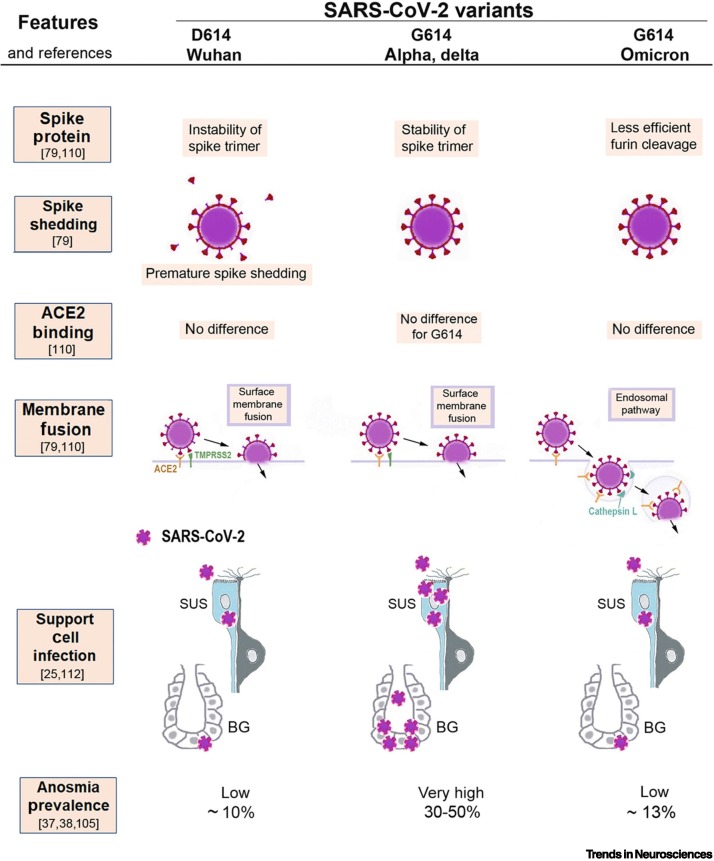
Figure 5.
Illustration of the molecular mechanisms that can explain why severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants cause different amounts of olfactory dysfunction.
This figure summarizes how the properties of three SARS-CoV-2 virus variants (D614, G614, and omicron) differ in ways that likely determine to what extent sustentacular support cells (SUS) in the olfactory epithelium become infected and whether their loss will lead to anosmia. The original D614 (Wuhan) virus results in premature spike shedding, lower spike density, and, therefore, less effective virus entry [79]. This may cause less infection of SUS and, therefore, results in a low prevalence of anosmia (~10%) [37]. The G614 variant has the D614G mutation, which stabilizes the spike trimer and prevents premature spike shedding; the higher spike density allows the G614 variant to infect SUS cells effectively [79], resulting in a high (30–50%) anosmia prevalence [38]. All three variants bind to the virus entry protein angiotensin-converting enzyme 2 (ACE2), expressed by SUS, and with no significant differences in binding affinity to ACE2 [21,79,80]; thus, this cannot explain differences in anosmia. The first two variants, D614 and G614, both enter host cells by using surface membrane fusion mediated by the protease TMPRSS2 [79,110]. The new mutations in the omicron variant cause a less efficient furin cleavage, resulting in reduced surface membrane fusion mediated by TMPRSS2 [79,80,111]. Therefore, omicron prefers an endosomal route that is less efficient for SUS infection, possibly because many host cells have developed defenses for the endosomal entry [78,79,111]. As a result, the omicron variant, despite retaining the D614G mutation, is associated with a lower anosmia prevalence of ~13% [105].