Abstract
Hoarding Disorder (HD) is a mental disorder characterized by persistent difficulties discarding or parting with possessions, often resulting in cluttered living spaces, distress, and impairment. Its etiology is largely unknown, but twin studies suggest that it is moderately heritable. In this study, we pooled phenotypic and genomic data from seven international cohorts (N = 27,651 individuals) and conducted a genome wide association study (GWAS) meta-analysis of parent- or self-reported hoarding symptoms (HS). We followed up the results with gene-based and gene-set analyses, as well as leave-one-out HS polygenic risk score (PRS) analyses. To examine a possible genetic association between hoarding symptoms and other phenotypes we conducted cross-trait PRS analyses. Though we did not report any genome-wide significant SNPs, we report heritability estimates for the twin-cohorts between 26–48%, and a SNP-heritability of 11% for an unrelated sub-cohort. Cross-trait PRS analyses showed that the genetic risk for schizophrenia and autism spectrum disorder were significantly associated with hoarding symptoms. We also found suggestive evidence for an association with educational attainment. There were no significant associations with other phenotypes previously linked to HD, such as obsessive-compulsive disorder, depression, anxiety, or attention-deficit hyperactivity disorder. To conclude, we found that HS are heritable, confirming and extending previous twin studies but we had limited power to detect any genome-wide significant loci. Much larger samples will be needed to further extend these findings and reach a “gene discovery zone”. To move the field forward, future research should not only include genetic analyses of quantitative hoarding traits in larger samples, but also in samples of individuals meeting strict diagnostic criteria for HD, and more ethnically diverse samples.
Subject terms: Genetics, Psychology
Introduction
Hoarding Disorder (HD) is one of the most recent mental disorders to be included in the DSM-5 [1] and ICD-11 [2]. Individuals with HD experience persistent difficulties parting with possessions, regardless of their value, due to a perceived need to save the items and distress associated with discarding them. This results in the accumulation of possessions that clutter active living areas and substantially compromise their intended use, causing clinically significant distress or impairment. Most people with HD also excessively acquire items that they do not need and experience distress if they are unable or are prevented from acquiring items (excessive acquisition specifier). Critically, these symptoms are not attributable to another medical or mental disorder, such as obsessive-compulsive disorder (OCD), psychosis or dementia [1, 2].
The prevalence of HD in the population is estimated to be approximately 1% to 2.5% for both men and women [3–6], but a much larger proportion of the population experience symptoms at various levels of severity, with estimates up to 6.7% [7] and 9% [8] in some studies. It is widely believed that the liability to hoarding symptoms (HS) is continuous in the general population, with clinically relevant HD at the extreme end of the spectrum [9].
HS typically appear in early-to-mid-adolescence and, in contrast to many other psychiatric disorders, symptom severity increases with age [10–13]. Psychiatric comorbidity is common in HD, with up to 70% of individuals having at least one additional disorder, most commonly anxiety and/or depression (DEP [14, 15]). Attentional problems are also common in individuals with HD [14, 16–18].
The etiology of HD is largely unknown, though likely to be multifactorial in nature and related to a complex interplay of genetic, neurobiological, and psychosocial factors. Family studies have consistently shown that HS run in families [15, 19–22]. Population-based twin studies have estimated the heritability of hoarding symptoms based on self-report questionnaires [3, 4, 7, 23–26]. In adults, heritability estimates range from 26 to 49%; the remaining variance was due to unique environmental factors and measurement error, whilst shared environmental factors appear to play a negligible role. In young people, large population-based samples of twins (N = 3974 twins, [3]; N = 25,523, [4]) reported higher heritability of hoarding symptoms amongst 15-year-old boys than in girls (33% vs 17%) and significant shared environment influences (22%) among female twins only, while Burton et al. [8] reported a heritability of 61% with no shared environment effect (221 twin pairs). Thus, it is possible that genetic and environmental influences on hoarding symptoms change across development, with shared environmental factors being more important in young people (particularly in girls).
Linkage and GWAS studies of HS have been rare thus far and have largely been conducted in small samples of OCD or Tourette syndrome patients. Candidate gene studies in individuals with OCD have suggested (largely non-replicated) associations between HS and a number of candidate variants [27–31]. Three previous modestly sized genome-wide linkage studies of HS in OCD or Tourette syndrome samples resulted in either no significant or conflicting results [21, 32, 33]. One study in OCD patients found linkage between HS and a region on chromosome 14q23-32 [21], and another linkage study in OCD patients found evidence for interaction with a region on chromosome 9q that houses SLC1A1, a glutamate transporter gene [32]. One GWAS of OCD symptom dimensions reported SETD3, a gene highly expressed in the brain and involved in apoptotic processes and transcriptomic changes, to be associated with HS [34]. Another GWAS focused on HS in a British twin cohort [35]. The sample included 3304 twins from the TwinsUK cohort, predominantly female (91.8%), with a mean age of 56.8 years. All participants completed the Hoarding Rating Scale-Self-Report (HRS-SR; [36]), a brief self-administered instrument consisting of five items (clutter, difficulty discarding, excessive acquisition, distress, and impairment). While no genome-wide significant loci were identified, two genomic loci on chromosomes 5 and 6 showed suggestive evidence for association with HS.
Larger samples are needed to increase power to detect significant genetic effects. Therefore, the current study aimed to conduct a GWAS meta-analysis of several large international cohorts from Sweden, the Netherlands, England, and Canada that included parent- or self-report hoarding scale data. We pooled data from seven population-based cohorts that together include 27,651 individuals (including 7012 twin-pairs), representing a more than eightfold increase in sample size compared to the previous study by Perroud et al. [35]. We followed up the results with gene-based and gene-set analyses, as well as leave-one-out hoarding symptom polygenic risk score (PRS) analyses and cross-trait PRS analyses to examine a possible genetic association between other phenotypes and HS.
Materials and methods
Cohorts and phenotype assessment
The Hoarding Symptom (HS) GWAS meta-analysis included individuals from seven different European-ancestry cohorts. Four cohorts are part of the Swedish Twin Registry (STR, [37]), namely different age groups of the Child and Adolescent Twin Study in Sweden (CATSS15, CATSS18, and CATSS24 [38]), and the Young Adult Twins in Sweden Study (YATSS [39]). CATSS is a prospective, longitudinal study of all twins born in Sweden since 1992. For CATSS, one measurement time point per individual was selected, preferring the measurement at age 24 over age 18 over age 15 if more than one measurement was completed. The other cohorts are from the Netherlands Twin Register (NTR, [40]), Spit for Science (SfS, [41, 42]), and TwinsUK (see Supplementary Material for detailed descriptions). Data from the TwinsUK cohort were included in a previous GWAS [35]. The cohorts are all population-based, predominantly including twins (except SfS), with a mean age-range between 11 and 57 (Table 1). Participants, or their parents, answered one of two questionnaires assessing HS.
Table 1.
Overview of cohorts included in the GWAS meta-analysis of HS.
| STR-CATSS15 | STR-CATSS18 | STR-CATSS24 | STR YATSS | NTR | SfS | TwinsUK | |
|---|---|---|---|---|---|---|---|
| N | 3605 | 3286 | 2313 | 2947 | 6839 | 5218 | 3443 |
| N MZ twin pairs | 241 | 256 | 191 | 552 | 866 | – | 317 |
| N DZ twin pairs | 1219 | 1137 | 685 | 348 | 425 | – | 775 |
| N siblings | – | – | – | – | 438 | – | – |
| N parents | – | – | – | – | 1361 | – | – |
| % female | 50% | 56% | 57% | 63% | 66% | 48% | 92% |
| Mean age ± SD | 15.47 ± 0.36 | 18.56 ± 0.33 | 23.84 ± 0.32 | 23.93 ± 1.78 | 41.49 ± 15.19 | 10.92 ± 2.79 | 56.7 ± 12.6 |
For each individual cohort included in the HS meta-analysis (STR-CATSS15, STR-CATSS18, STR-CATSS24, STR-YATSS, NTR, SfS, TwinsUK), the table lists the total sample size included (N), the number of monozygotic twin pairs (N MZ twin pairs), the number of dizygotic twin pairs (N DZ twin pairs), the number of siblings (N siblings), the number of parents (N parents), the percentage of females and males in the total N (% females (males)), and the mean age with standard deviations (SD). Twins where only one twin participated were not counted as twins. NTR twin pairs include 10 multiplets, (31 individuals), 185 spouses of twin probands and 1153 twins without any other family member participating. Note that CATSS samples were later pooled across the three cohorts (CATSS15, CATSS18, CATSS24) for GWAS analysis, depending on the platform they were genotyped on (GSA, PsychChip).
In STR, NTR, and TwinsUK, HS were assessed using four (STR-YATSS, STR-CATSS24) to five items (STR-CATSS15, STR-CATSS18, NTR, TwinsUK) of the Hoarding Rating Scale Self-Report (HRS-SR; [43]), while in SfS parent- or self-reported hoarding traits were assessed using two items from the Toronto Obsessive Compulsive Scale (TOCS), a 21-item questionnaire described elsewhere [44, 45] (see Supplementary Table S1 for questionnaire details). Though the TOCS was originally designed to measure obsessive-compulsive symptoms, the two questions used here effectively reflect two core components of hoarding, namely acquisition of objects and difficulty discarding. To summarize across HRS-SR items, four items of the HRS-SR were used to calculate a one-factor model using a latent variable analysis with the R package lavaan [46] confirmatory-factor-analysis function. For STR item 4 of the HRS-SR was not assessed (“To what extent do you experience impairment in your life (daily routine, job/school, social activities, family activities, financial difficulties) because of clutter, difficulty discarding, or problems with buying or acquiring things?”), while for NTR and TwinsUK item 5 was not included (“To what extent do you experience emotional distress because of clutter, difficulty discarding or problems with buying or acquiring things?”). Fit indices of the one-factor model were compared to ensure that by dropping the respective item, still the same construct is measured for all cohorts. Standardized individual factor scores were calculated for the common factor model using the lavPredict function. Standardized individual factor scores were calculated for the common factor model using the lavPredict function. In case an individual was missing one item, the mean of the remaining three items was used to impute the missing value. If more than one item was missing, the individual was removed from the analysis. The two SfS Hoarding items of the TOCS were summed and standardized into a Z-score. To ensure reliable and valid symptom reporting, SfS participants <12 years of age with self-reported HS were excluded.
Genome-wide association analysis
All participants were genotyped on SNP-arrays based on DNA from saliva or blood. One part of the STR-CATSS samples was genotyped on the PsychChip genotyping array (N = 8598), another part was genotyped on the GSA genotyping array (N = 606). For the GWAS analyses, the STR-CATSS cohorts (CATSS15, CATSS18, CATSS24) were pooled over each genotyping platform (GSA, PsychChip), forming two separate CATSS datasets (STR-CATSS-GSA and STR-CTASS-PC). Each of the six datasets (STR-CATSS-GSA, STR-CATSS-PC, STR-YATSS, NTR, SfS, and TwinsUK) underwent stringent quality-control (QC), including the removal of non-European ancestry outliers based on PCA and imputation using the HRC [47] (STR, NTR) or the 1000 G [48] (SfS, TwinsUK) reference sets (see Supplementary Material for more details). After genotyping, quality control, and imputation of each cohort, STR included 12,151, NTR 6839, SfS 5218, and TwinsUK 3443 (total N = 27,651) individuals with complete genotypic and phenotypic information.
A linear mixed modeling GWAS was conducted within each cohort using GCTA-fastGWA [49, 50]. For STR, NTR, and TwinsUK a sparse Genetic Relatedness Matrix (GRM) was calculated and the first 10 principal components, sex, age, age squared, and genotyping batches were used as covariates. In a sparse GRM all off-diagonal values below 0.05 are set to 0, thereby accounting for the close relatedness of individuals in the data. For SfS, analyses were performed on unrelated individuals; the first enrolled sibling from each family was selected for further analysis. The analysis was performed with a full GRM and sex, age, respondent (parent vs. child reporting), genotyping array, principal components 1–3 and projected principal components 1–3 (see Supplement for details) as covariates.
Next, the resulting GWAS summary statistics were cleaned and harmonized. All variants were filtered on minor allele frequency (MAF) > 1%, and imputation-quality score > 0.8. All datasets were aligned to the HRC-reference. In case alleles were reported on different strands, they were flipped to the orientation in the HRC reference. Strand ambiguous A/T and C/G SNPs were removed if their MAF was ≥0.4. Remaining ambiguous SNPs were strand aligned by comparing MAF to the HRC reference [47]. We then used METAL [51] within the Rapid Imputation for COnsortias PIpeLIne (Ricopili) [52] to conduct an inverse variance weighted meta-analysis. The genomic control factor (Lambda and Lambda1000) was inspected for each individual cohort to detect any residual population stratification or systematic technical artifacts. Also, the linkage disequilibrium (LD) score regression (LDSC) [53] intercept was inspected as an alternative measure of test statistic inflation. The genome-wide significance threshold was set at 5 × 10–8.
Heritability
Heritability estimates of each individual cohort were extracted from the GCTA association output. GCTA uses a restricted maximum likelihood (REML) approach [54] to estimate heritability in the GRM that is supplied to correct for relatedness in the linear association test. This means that for the twin cohorts (STR, NTR, and TwinsUK), the heritability was based on the sparse GRM, while for the unrelated SfS cohort, heritability was based on the full GRM. For all heritability estimates, the same covariates as in the GWAS analyses were used. We further used LDSC [53] to calculate the SNP heritability of the HS GWAS meta-analysis. The SNP heritability in LDSC is based on the estimated slope from the regression of the SNP effect sizes from the GWAS on the LD score.
Gene-based and gene-set analyses
We carried out a Multi-marker Analysis of GenoMic Annotation (MAGMA) [55] v1.08 as implemented in the web-based tool Functional Mapping and Annotation of Genome-Wide Association Studies (FUMA) [56] v1.3.7 to test genetic associations at the gene level for the combined effect of SNPs in or near protein coding genes. Gene-based p-values were computed by mapping SNPs to their corresponding gene(s) based on their position in the genome. Positional mapping was based on ANNOVAR annotations and the maximum distance between SNPs and genes was set to the default setting of 10 kb. Based on the results of gene analysis, competitive gene-set analysis was performed with default parameters. The 15,496 gene-sets were obtained from MsigDB v7.0, including ‘Curated gene sets’ consisting of nine data resources including KEGG, Reactome, and BioCarta, and GO terms consisting of three categories (biological processes, cellular components, and molecular functions).
Cross-trait polygenic risk score (PRS) analyses
To explore the genetic relationship between HS and other phenotypes, we calculated a range of PRSs based on large-scale GWAS summary statistics. We selected mainly studies of psychiatric disorders, i.e., OCD [57], DEP [58], schizophrenia (SCZ) [59], autism spectrum-disorder (ASD) [60], attention-deficit-hyperactivity disorder (ADHD) [61], and educational attainment (EA) [62]. PRS were computed in PRSice2 [63] for each cohort. The PRS scores were calculated as the weighted sum of the risk allele dosages at pre-selected p-value thresholds based on the reported thresholds in the primary publications (EA: P = 1; ADHD, ASD, OCD, SCZ: P = 0.01; DEP: P = 0.5). For STR, PRS analyses were conducted separately for the three datasets (STR-CATSS-GSA, STR-CATSS-PC, STR-YATSS) and were subsequently merged.
To evaluate the relationship between each PRS score and HS in every cohort, we employed generalized estimating equations (GEE) in R (STR, NTR, and TwinsUK). The GEE analysis accounts for the relatedness in the datasets. As SfS did not contain any related individuals, we carried out linear regression, as implemented within the PRSice2 pipeline. Again, the same covariates that were previously used in the respective GWASs were included.
PRS estimates per discovery phenotype were summarized across all target datasets by means of an inverse variance meta-analysis using the metagen package in R. We examined heterogeneity in PRS estimates across the cohorts with Cochran’s Q test [64] and Higgin’s I² [65, 66]. Q is calculated as the weighted sum of the squared differences between individual cohort effects and the pooled effect across cohorts, with the weights being those used in the pooling method. The I² statistic describes the percentage of variation across studies that is due to heterogeneity rather than sample variation and does, unlike Q, not inherently depend upon the number of measures included in the meta-analysis. Regardless of observed heterogeneity, we calculated a fixed effects model to evaluate the association of each PRS with HS. If there was considerable observed heterogeneity across study sites (I² > 0.5 and/or PQ < 0.05), we further calculated a random effects model.
Compatibility of cohorts
To identify if the summary statistics from any of the included cohorts substantially deviated from the others, we performed leave-one-out (LOO) GWAS meta-analyses and carried out a set of sensitivity analyses. With the replication module of the Ricopili pipeline, sign tests on the top SNPs (inclusion threshold of p = 0.0001, p = 0.00001, and p = 0.000001) were performed between each pair-wise combination of cohorts as well as between LOO meta-analyses and the left-out cohort to identify any cohort in which the GWAS results markedly deviated from the rest of the cohorts. Sign-tests allow for the quantification of independent genomic regions that have the same direction of effect in two separate summary statistics. The output, in form of a ratio, gives an estimate of the percentage of genomic regions with the same direction of effect in the two compared datasets. A sign-test is a binomial test with the null-hypothesis = 0.5, with a ratio above 0.5 indicating convergence, and a ratio below 0.5 indicating divergence. While certain fluctuations in the sign-tests across different p-value thresholds are expected, depending on the true association of each SNP with the phenotype, we mainly aimed to assess whether a specific cohort or age-group markedly deviated from the rest.
To evaluate the relationship between the PRS scores of each LOO GWAS and standardized HS scores in the left-out cohort, we conducted LOO PRS analyses, following the same procedure as for the cross-trait PRS analyses described above (see previous method-section on cross-trait PRS analyses for details).
Results
Phenotype normalization
The distributions of each cohorts’ sum-scores have been maximally normalized (distribution of the item- and total raw hoarding scores are shown in Supplementary Figs. S1–S6, distribution of the one-factor model scores (STR, NTR, and TwinsUK) and standardized scores (SfS) are shown in Supplementary Figs. S7–S10). An over-representation of zero sum-scores indicates censoring. The model-fit of the one-factor models in the latent variable analysis of the HRS-SR for NTR (CFI = 0.999, SRMR = 0.014) and STR (STR-GSA: CFI = 0.995, SRMR = 0.032; STR-PC: CFI = 0.997, SRMR = 0.020; STR-YATSS: CFI = 0.997, SRMR = 0.020) were excellent, thereby demonstrating that all items of the HRS-SR highly load onto one common factor and psychometrically measure the same construct, allowing us to drop one item of the HRS-SR per analysis. The two TOCS hoarding items in the SfS data could not be assessed for model fit, however, the two items were highly comparable in wording to items 2 and 3 of the HRS.
Genome-wide association results
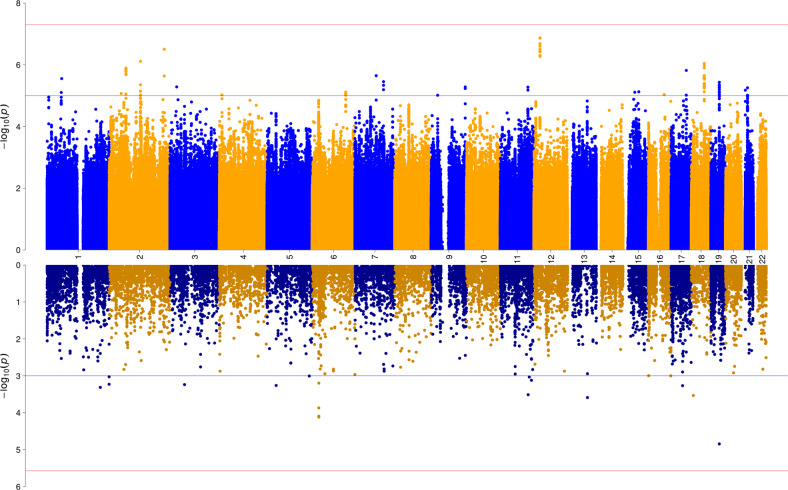
The final dataset included 27,651 individuals with complete phenotypic and genotypic data and 6,541,342 autosomal SNPs. No significant inflation was observed (λ = 1.024, λ1000 = 1.001, LDSC intercept = 1.0173, see Supplementary Fig. S11 for QQ plot). No SNP exceeded the genome-wide significance threshold (see Fig. 1 for a Miami-plot including the Manhattan-plot of the GWAS in the upper panel). The SNPs with the lowest p-values (<1 × 10–6) were rs117321479 (P = 1.36 × 10–7) on chromosome 12, rs78426839 (P = 3.12 × 10–7) and rs7567224 (P = 7.70 × 10–7) on chromosome 2, and rs72927972 (P = 9.09 × 10–7) on chromosome 18 (see Supplementary Figs. S12–S15 for regional association plots and forest plots). The region tagged by rs117321479 spans 57.6 kb (LD r² > 0.6) and entails the gene SOX5. The region tagged by rs78426839 spans 22.1 kb (LD r² > 0.6) and entails the genes TUBA4B, DNAJB2, PTPRN, MIR153-1, RESP18, and DNPEP. The region tagged by rs7567224 spans 24.40 kb (LD r² > 0.6) and entails the gene CNTNAP5, while the region tagged by rs78426839 spans 94.3 kb (LD r² > 0.6). In addition, 19 independent SNPs with p < 1 × 10–5 were identified (see Supplementary Table S2 for a list of corresponding association results).
Fig. 1. Miami plot of the association results from the GWAS meta-analysis (upper panel) and of the gene-wide association analysis (lower panel) of HS.
The y-axes represent −log10 P values for the association of SNPs/genes with HS. The x-axis represents chromosomes 1 to 22. In the upper plot, the P-value threshold for genome-wide significance (5 × 10–08) is represented by the horizontal red line, suggestive significance (p = 1 × 10–05) by the blue line. In the lower panel, Bonferroni-corrected gene-wide significance (p = 2.682 × 10–06) is represented by the horizontal red line, suggestive gene-wide significance (p = 1 × 10–03) is indicated by the blue horizontal line.
Heritability
For the twin cohorts, the additive genetic variance of HS, estimated based on the sparse genetic relatedness matrices, ranged between 0.26 (NTR) and 0.48 (TwinsUK), with estimates for the STR cohorts in between (STR-CATSS15: 0.47, STR-CATSS18: 0.29, STR-CATSS24: 0.35, STR-YATSS = 0.28). Note that the SNP-heritability estimates based on the twin cohorts are largely driven by the twin resemblance (~0.5 between DZ twins and siblings, 1.0 for MZ twins, and 0 between unrelated individuals). The heritability for SfS, only including unrelated individuals, was 0.11 (SE = 0.057, P = 0.0303). The SNP-based heritability estimate of the GWAS meta-analysis using LDSC resulted in a total observed-scale heritability of 0.019 (SE = 0.016, Z = 1.18, P = 0.235).
Gene-based analyses
We conducted gene-based tests to determine whether any protein-coding gene carries a load of common variation associated with HS. SNPs were mapped to 18,646 protein coding genes obtained from Ensembl build 85. No gene reached the Bonferroni-corrected significance threshold of p = 0.05/18,646 = 2.682 × 10–6 (see Fig. 1 for a Miami-plot including the Manhattan-plot of the gene-based test in the lower panel, and Supplementary Fig. S11 for a QQ-plot). Also, none of 15,483 tested gene-sets reached the Bonferroni-corrected significance threshold.
Cross-trait PRS analyses
To evaluate the genetic overlap between HS and other potentially related phenotypes of HS, we conducted PRS analyses. Publicly available summary statistics of OCD, DEP, SCZ, ASD, ADHD, and EA served as discovery datasets, with each HS cohort as the target dataset. GEE (STR, NTR, TwinsUK) and linear regression analysis (SfS) revealed Bonferroni-corrected significant (p < 0.05/7 = 0.00714) associations between HS and PRSs based on all DEP, SCZ and EA, though not consistently across all target cohorts (see Supplementary Table S3). In a meta-analysis summarizing PRS results across all HS cohorts, the PRS for SCZ showed a significant association with HS (Pfixed = 2.43 × 10–06, Prandom = 0.00422). When not taking the heterogeneity of the individual PRS estimates into account, the meta-analyzed PRSs of ASD and EA also showed significant associations with HS (ASD: Pfixed = 0.00426; EA: Pfixed = 1.15E−05). As the associations with the PRS of EA showed substantial (I² = 0.76598, PQ = 0.00504) heterogeneity, we further conducted random effects models to examine if the association with HS remains significant when accounting for this heterogeneity, resulting in a nominally significant association (EA: Prandom = 0.00765). The associations with the PRS of DEP, OCD, and ADHD did not show any significant associations with HS in the meta-analysis (see Table 2 for all results).
Table 2.
Depicted are results from the PRS meta-analysis for HS (leave-one-out), obsessive-compulsive disorder (OCD), depressive disorder (DEP), schizophrenia (SCZ), autism-spectrum disorder (ASD), attention-deficit hyperactivity disorder (ADHD), and educational attainment (EA) for pre-selected p-value thresholds (Pthreshold), meta-analyzed across all target datasets (STR, NTR, SfS, TwinsUK).
| Discovery | Pthreshold | Neffective | Heterogeneity | Pfixed | Prandom | |
|---|---|---|---|---|---|---|
| I² | PQ | |||||
| Hoarding Symptoms (Leave-one-out) | ||||||
| HS | 0.5 | 25,389 | 0 | 0.925 | 0.553 | |
| Cross-trait | ||||||
| OCD | 0.1 | 25,465 | 0 | 0.629 | 0.674 | |
| DEP | 0.05 | 25,606 | 0.827 | 0.001 | 0.015 | 0.586 |
| SCZ | 0.1 | 25,547 | 0.450 | 0.142 | 2.43E−06 | |
| ASD | 0.1 | 25,977 | 0.267 | 0.252 | 0.004 | |
| ADHD | 0.1 | 25,938 | 0.555 | 0.081 | 0.213 | 0.816 |
| EA | 1 | 25,307 | 0.766 | 0.005 | 1.15E−05 | 0.008 |
As measures of heterogeneity of PRS associations across all target datasets, Higgin’s I² statistic and the p-value for Cochran’s Q test (PQ) are reported. Pfixed and Prandom list the p-values of a fixed and a random-effects model, respectively. A random effects model was only calculated if there was substantial (I² > 0.5 and/or PQ < 0.05) heterogeneity across the datasets. The effective sample size (Neffective) is summed over the effective sample size of every target dataset. For STR, NTR, and TwinsUK the effective N was determined based on the actual N (including family members) weighted by the ratio of the squared SE from the GEE sandwich-corrected model and the naive model (no correction), for SfS the total sample N was used. Bonferroni-corrected significant p-values (<0.05/7 = 0.00714) are in bold.
Compatibility of cohorts
No genome-wide significant heterogeneity was observed in the HS GWAS meta-analysis (see Supplementary Fig. S16 for Manhattan-plot and QQplot of the heterogeneity test). A range of sensitivity analyses, including LOO GWAS analyses (see Supplementary Figs. S17–S20 for LOO Manhattan plots and QQ-plots), and subsequent sign-test analyses and LOO PRS analyses provided further evidence that there was no systematic and substantial heterogeneity across the different cohorts.
First, we conducted sign tests at three different p-value thresholds (1 × 10–06, 1 × 10–05, 1 × 10–04) between each pair-wise combination of STR, NTR, SfS, and TwinsUK (see Supplementary Table S4). Second, to determine if there was any pronounced age-related effect in the STR or NTR data, we conducted sign-test analyses between LOO GWAS analyses of age-separated STR sub-cohorts (Supplementary Table S5), age-separated NTR sub-cohorts (Supplementary Table S6) and the respective left-out cohort. The STR cohorts were divided into four age groups, pertaining to their division into separate phenotyping rounds (CATSS15 mean age = 15.46; CATSS18 mean age = 18.56; CATSS24 mean age = 23.84; YATSS mean age = 23.93), while the NTR data was separated into three age groups (group1 < 30 years; group2 30–45 years; group3 > 45 years). The sign test results at the p-value threshold of p = 1 × 10–06 identified very few independent genomic regions (0–6) and are therefore difficult to interpret. While the ratios of the sign tests for the other two p-value thresholds (p = 1 × 10–05 and p = 1 × 10–04) varied between 0.2 and 0.8, there is no apparent pattern indicating a systematic deviation of one cohort from the rest.
The LOO PRS analyses did not show a significant association between any of the PRSs and the HS score of the left-out cohort. While this could suggest heterogeneity across the cohorts, it is likely indicating a low power for this analysis considering that each discovery cohort had a rather low sample size and we only saw significant associations with PRS based on GWASs with considerably higher sample sizes (SCZ, DEP, EA). See Supplementary Tables S3 and 2 for results.
Discussion
With 27,651 included individuals we conducted the largest GWAS study of HS in the population to date. Although we could not report any genome-wide significant SNPs, we found a significant contribution of common genetic factors to HS as indicated by a substantial genetic SNP heritability of 11% (P = 0.0303) in one of our cohorts (SfS) with unrelated samples. It suggests that, with sufficient power, specific genetic variants that are associated with HS will be eventually identified. SNP-based heritability of the meta-analysis as calculated with LDSC was low (h² = 0.019, SE = 0.016, Z = 1.18) and non-significant. We therefore did not conduct genetic correlation analyses as it is suggested to have a heritability Z-score of above 1.5 (optimal > 4) in order for the analysis to be meaningful [53, 67]. We also found a significant genetic variance component in the twin family cohorts, ranging from 28 to 48%. These heritability estimates may be largely driven by the relatedness between the samples and are indeed more comparable to pedigree-based twin-heritability estimates. Ivanov et al. [4] reported twin-heritability estimates of 41%, 31%, and 29% for the Swedish CATSS15, CATSS18, and YATSS cohorts, respectively, while in this study we report heritability estimates of 47%, 29%, and 28% for the same cohorts, respectively.
The region tagged by the SNP with the lowest p-value in our analysis (rs117321479) entails the gene SOX5, which is a member of the SOX family of transcription factors involved in the regulation of embryonic development and in the determination of cell fate [68]. SOX5 has been identified as a gene with a high pleiotropic effect on a broad spectrum of psychiatric disorders and has been associated especially with ASD, BIP, MD, and SCZ, and to a lesser degree also with OCD, TS, ADHD, and AN [69]. Overall, SOX5 has been genome-wide significantly associated with 64 phenotypes, spanning a wide range of domains including psychiatric (e.g. neuroticism [70, 71]), skeletal (e.g. height [72]), reproductive (e.g. age at menarche [73]), metabolic (e.g. hip circumference, BMI [70]), and environmental traits (e.g. household income [70], educational attainment [62]). Given this possible pleiotropy, it suggests that SOX5 might be involved in susceptibility to general psychopathology.
By current standards [58–61], the size of our GWAS was modest and no significant effects were observed in the leave-one-sample-out analysis of PRS associated with HS, indicating that the lack of finding genome-wide hits is most likely attributable to a lack of power. We further observed a rather high number of individuals (28.8%) with zero scores on the hoarding rating scales, leading to a relatively low variance of symptom scores in our dataset, and our datasets included a high number of twins, both of which likely reduced the effective sample size and contributed to the power issue.
Another reason why we did not find a significant signal may lie in differences between the included study cohorts, beyond any heterogeneity that we were able to detect. Possible sources of heterogeneity across the datasets include age, instruments used to assess HS, or ascertainment of data. The SfS cohort stands out compared to the other cohorts with regards to age and questionnaire used. We addressed this issue by applying a framework of sensitivity and heterogeneity analyses. Neither in the genome-wide heterogeneity test, nor in the sign-tests or LOO PRS analyses did we observe a pattern that indicated a systematic deviation of one cohort from the rest. We therefore concluded that a meta-analysis of the cohorts is warranted and that the lack of signal is indeed most likely attributable to a lack of power. As most of the individuals included in the cohorts in this study were relatively young compared to the age when the full disorder debuts, there is the possibility that HS reported by younger individuals are the outcome of a somewhat different phenotype than HS reported at an older age.
Cross-trait PRS analyses showed significant results. The genetic risk for schizophrenia was significantly associated with HS, while we found suggestive evidence for an association with autism spectrum disorder and educational attainment. This suggests that a well-powered GWAS such as those used in the cross-trait PRS analyses (SCZ: Neffective = 214,576; ASD: Neffective = 44,367; EA: Neffective = 245,621) can pick up genetic signals that may be associated with hoarding symptoms. We consider this a further indicator that additional power is needed to obtain reliable signal in a quantitative trait GWAS for HS. The lack of significant associations with the most described comorbidities in HD (OCD, depression, anxiety, ADHD) was unexpected and possibly attributable to the currently modest size of the discovery samples.
Previous twin studies of HS have shown a clear role of genetic factors [3, 4, 7, 23–26]. The current study confirmed these observations with a significant SNP-based heritability of HS. It is not entirely surprising that we did not discover any genome-wide significant associations, as other studies with similar sample sizes that investigated quantitative measures of symptoms also lacked power to discover associations [74, 75]. Nevertheless, the quantitative ADHD symptom GWAS by Middeldorp et al. [75] showed a high genetic correlation and strong concordance at individual loci with clinical ADHD [61], thereby supporting the hypothesis that clinically diagnosed cases are the extreme of a quantitative symptom trait and further demonstrating the usefulness and importance of quantitative assessment of symptoms in the population.
A further limitation is that hoarding symptom scales may reflect heterogeneous disorders. It is known that many different psychiatric disorders can cause hoarding-like symptoms, such as schizophrenia, OCD, or severe depression. HD is essentially a diagnosis of exclusion (DSM-5, ICD-11). As in any study based on self-administered instruments, it was not possible to rule out these other causes of HS. It will further be of interest to determine the extent to which HS in the population and clinical HD share the same genetic susceptibility. However, no case-control GWAS of HD exists, nor is HD assessed in large datasets like UK BioBank [76, 77], which is unfortunate given the relatively high prevalence of around 2.5% for HD [6] and high individual and societal cost [78, 79]. Thus, for the time being, the study of HS in the population may be the only feasible approach to understanding the genetics of HD.
To conclude, we found that HS are heritable, confirming and extending previous twin studies. Nevertheless, we had limited power to detect any genome-wide significant loci. Much larger samples will be needed to further extend our findings and reach a “gene discovery zone”. Further, additional samples should be more ethnically diverse to ensure that results are relevant to individuals of non-European ancestry [80]. Future research should include the collection of DNA samples from individuals with HS, as well as samples from strictly diagnosed HD patients.
Supplementary information
Acknowledgements
We acknowledge The Swedish Twin Registry (STR) for data access. The STR is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no 2017-00641. The computations/data handling were enabled by resources provided by the Swedish National Infrastructure for Computing (SNIC) at Uppmax partially funded by the Swedish Research Council through grant agreement no. 2018-05973. The Netherlands Twin Register (NTR) warmly thanks all twin families for their participation. NTR is supported by multiple grants from the Netherlands Organisations for Scientific Research (NWO) and Medical Research (ZonMW): Netherlands Twin Registry Repository (NWO 480-15-001/674); the Biobank-based integrative omics study (BIOS) funded by BBMRI-NL (NWO projects 184.021.007 and 184.033.111); the European Science Council (ERC) Genetics of Mental Illness (ERC Advanced, 230374, PI Boomsma); the Royal Netherlands Academy of Science Professor Award (PAH/6635) to DIB; Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06), the Avera Institute, Sioux Falls, South Dakota (USA) and the National Institutes of Health (NIH R01 HD042157-01A1). Part of the genotyping was funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health and Grand Opportunity grants (1RC2 MH089951). TwinsUK is funded by the Wellcome Trust, Medical Research Council, Versus Arthritis, European Union Horizon 2020, Chronic Disease Research Foundation (CDRF), Zoe Ltd, the National Institute for Health and Care Research (NIHR) Clinical Research Network (CRN) and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. Spit for Science was supported by the Canadian Institutes of Health Research (RJS, MOP‐93696 and PDA, MOP‐106573). Dr. Arnold is supported by the Alberta Innovates Translational Health Chair in Child and Youth Mental Health. We acknowledge support by the Open Access Publication Fund of Humboldt-Universität zu Berlin.
Author contributions
NIS, DJAS, DC, DMC, DIB, and MM contributed to the conception of the over-all study design; CLB, RP, ML, JJC, JJH, VZI, HL, PL, PM, CR, RJS, HMW, JC, PDA, DIB, SMM, DMC, and DC contributed to the data collection of the individual datasets and/or provided code; NIS, DJAS, TS, and CI conducted all primary data analyses; NIS, DS, and DMC drafted the manuscript; all authors provided critical edits and discussions and approved the submitted version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The meta-analyzed summary statistics will be made available via the Psychiatric Genomics Consortium Download page (https://www.med.unc.edu/pgc/download-results/).
Competing interests
David Mataix-Cols receives royalties for contributing articles to UpToDate, Wolters Kluwer Health, outside of the submitted work. Henrik Larsson reports receiving grants from Shire Pharmaceuticals; personal fees from and serving as a speaker for Medice, Shire/Takeda Pharmaceuticals and Evolan Pharma AB; and sponsorship for a conference on attention-deficit/hyperactivity disorder from Shire/Takeda Pharmaceuticals and Evolan Pharma AB, all outside the submitted work. Henrik Larsson is editor-in-chief of JCPP Advances. Russell J. Schachar has consulted to E. Lilly, Highland Therapeutics and eHave. All other authors report no potential conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nora I. Strom, Dirk J. A. Smit.
These authors jointly supervised this work: David Mataix-Cols, Danielle Cath.
Change history
12/22/2022
A Correction to this paper has been published: 10.1038/s41398-022-02288-z
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-02248-7.
References
- 1.DSM-5. Diagnostic and statistical manual of mental disorders: DSM-5. 2013;237–42. [DOI] [PubMed]
- 2.World Health Organization. ICD-11: International classification of diseases (11th revision). Retrieved fromhttps://www.icd.who.int/ 2019.
- 3.Ivanov VZ, Mataix-Cols D, Serlachius E, Lichtenstein P, Anckarsäter H, Chang Z, et al. Prevalence, comorbidity and heritability of hoarding symptoms in adolescence: a population based twin study in 15-year olds. PLoS ONE. 2013;8:1–7. doi: 10.1371/journal.pone.0069140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov VZ, Nordsletten A, Mataix-Cols D, Serlachius E, Lichtenstein P, Lundström S, et al. Heritability of hoarding symptoms across adolescence and young adulthood: A longitudinal twin study. PLoS ONE. 2017;12. 10.1371/JOURNAL.PONE.0179541. [DOI] [PMC free article] [PubMed]
- 5.Nordsletten AE, Reichenberg A, Hatch SL, Fernández De La Cruz L, Pertusa A, Hotopf M, et al. Epidemiology of hoarding disorder. Br J Psychiatry. 2013;203:445–52. doi: 10.1192/bjp.bp.113.130195. [DOI] [PubMed] [Google Scholar]
- 6.Postlethwaite A, Kellett S, Mataix-Cols D. Prevalence of Hoarding Disorder: a systematic review and meta-analysis. J Affect Disord. 2019;256:309–16. doi: 10.1016/j.jad.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Mathews CA, Delucchi K, Cath DC, Willemsen G, Boomsma DI. Partitioning the etiology of hoarding and obsessive-compulsive symptoms. Psychol Med. 2014;44:2867–76. doi: 10.1017/S0033291714000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton CL, Park LS, Corfield EC, Forget-Dubois N, Dupuis A, Sinopoli VM, et al. Heritability of obsessive–compulsive trait dimensions in youth from the general population. Transl Psychiatry. 2018;8:191. doi: 10.1038/s41398-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timpano KR, Broman-Fulks JJ, Glaesmer H, Exner C, Rief W, Olatunji BO, et al. A taxometric exploration of the latent structure of hoarding. Psychol Assess. 2013;25:194–203. doi: 10.1037/a0029966. [DOI] [PubMed] [Google Scholar]
- 10.Ayers CR, Saxena S, Golshan S, Wetherell JL. Age at onset and clinical features of late life compulsive hoarding. Int J Geriatr Psychiatry. 2010;25:142–9. doi: 10.1002/gps.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cath DC, Nizar K, Boomsma D, Mathews CA. Age-specific prevalence of hoarding and obsessive compulsive disorder: a population-based study. Am J Geriatr Psychiatry. 2017;25:245–55. doi: 10.1016/j.jagp.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisham JR, Frost RO, Steketee G, Kim HJ, Hood S. Age of onset of compulsive hoarding. J Anxiety Disord. 2006;20:675–86. doi: 10.1016/j.janxdis.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov VZ, Mataix-Cols D, Serlachius E, Brander G, Elmquist A, Enander J, et al. The developmental origins of hoarding disorder in adolescence: a longitudinal clinical interview study following an epidemiological survey. Eur Child Adolesc Psychiatry. 2021;30:415–25. doi: 10.1007/s00787-020-01527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost RO, Steketee G, Tolin DF. Comorbidity in hoarding disorder. Depress Anxiety. 2011;28:876–84. doi: 10.1002/da.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertusa A, Fullana MA, Singh S, Alonso P, Menchón JM, Mataix-Cols D. Compulsive hoarding: OCD symptom, distinct clinical syndrome, or both? Am J Psychiatry. 2008;165:1289–98. doi: 10.1176/appi.ajp.2008.07111730. [DOI] [PubMed] [Google Scholar]
- 16.Fullana MA, Vilagut G, Mataix-Cols D, Adroher ND, Bruffaerts R, Bunting B, et al. Is ADHD in childhood associated with lifetime hoarding symptoms? An epidemiological study. Depress Anxiety. 2013;30:741–8. doi: 10.1002/da.22123. [DOI] [PubMed] [Google Scholar]
- 17.Hartl TL, Duffany SR, Allen GJ, Steketee G, Frost RO. Relationships among compulsive hoarding, trauma, and attention-deficit/hyperactivity disorder. Behav Res Ther. 2005;43:269–76. doi: 10.1016/j.brat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Tolin DF, Villavicencio A. Inattention, but not OCD, predicts the core features of hoarding disorder. Behav Res Ther. 2011;49:120–5. doi: 10.1016/j.brat.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost RO, Gross RC. The hoarding of possessions. Behav Res Ther. 1993;31:367–81. doi: 10.1016/0005-7967(93)90094-B. [DOI] [PubMed] [Google Scholar]
- 20.Samuels J, Joseph Bienvenu O, Riddle MA, Cullen BAM, Grados MA, Liang KY, et al. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther. 2002;40:517–28. doi: 10.1016/S0005-7967(01)00026-2. [DOI] [PubMed] [Google Scholar]
- 21.Samuels J, Yin YS, Grados MA, Willour VL, Bienvenu OJ, Greenberg BD, et al. Significant linkage to compulsive hoarding on chromosome 14 in families with obsessive-compulsive disorder: results from the OCD Collaborative Genetics Study. Am J Psychiatry. 2007;164:493–9. doi: 10.1176/ajp.2007.164.3.493. [DOI] [PubMed] [Google Scholar]
- 22.Steketee G, Kelley AA, Wernick JA, Muroff J, Frost RO, Tolin DF. Familial patterns of hoarding symptoms. Depress Anxiety. 2015;32:728–36. doi: 10.1002/da.22393. [DOI] [PubMed] [Google Scholar]
- 23.Iervolino AC, Perroud N, Fullana MA, Guipponi M, Cherkas L, Collier DA, et al. Prevalence and heritability of compulsive hoarding: a twin study. Am J Psychiatry. 2009;166:1156–61. doi: 10.1176/appi.ajp.2009.08121789. [DOI] [PubMed] [Google Scholar]
- 24.Iervolino AC, Rijsdijk FV, Cherkas L, Fullana MA, Mataix-Cols D. A multivariate twin study of obsessive-compulsive symptom dimensions. Arch Gen Psychiatry. 2011;68:637–44. doi: 10.1001/archgenpsychiatry.2011.54. [DOI] [PubMed] [Google Scholar]
- 25.López-Solà C, Fontenelle LF, Alonso P, Cuadras D, Foley DL, Pantelis C, et al. Prevalence and heritability of obsessive-compulsive spectrum and anxiety disorder symptoms: a survey of the Australian Twin Registry. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:314–25. doi: 10.1002/ajmg.b.32233. [DOI] [PubMed] [Google Scholar]
- 26.Taylor S, Jang KL, Asmundson GJG. Etiology of obsessions and compulsions: a behavioral-genetic analysis. J Abnorm Psychol. 2010;119:672–82. doi: 10.1037/a0021132. [DOI] [PubMed] [Google Scholar]
- 27.Alonso P, Gratacòs M, Menchón JM, Segalàs C, González JR, Labad J, et al. Genetic susceptibility to obsessive-compulsive hoarding: the contribution of neurotrophic tyrosine kinase receptor type 3 gene1. Genes, Brain Behav. 2008;7:778–85. doi: 10.1111/j.1601-183X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 28.Lochner C, Kinnear CJ, Hemmings SMJ, Seller C, Niehaus DJH, Knowles JA, et al. Hoarding in obsessive-compulsive disorder: clinical and genetic correlates. J Clin Psychiatry. 2005;66:1155–60. doi: 10.4088/JCP.v66n0911. [DOI] [PubMed] [Google Scholar]
- 29.Sinopoli VM, Erdman L, Burton CL, Park LS, Dupuis A, Shan J, et al. Serotonin system genes and hoarding with and without other obsessive–compulsive traits in a population-based, pediatric sample: a genetic association study. Depress Anxiety. 2020;37:760–70. doi: 10.1002/da.22996. [DOI] [PubMed] [Google Scholar]
- 30.Timpano KR, Schmidt NB, Wheaton MG, Wendland JR, Murphy DL. Consideration of the BDNF gene in relation to two phenotypes: hoarding and obesity. J Abnorm Psychol. 2011;120:700–7. doi: 10.1037/a0024159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:408–16. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang KY, Wang Y, Yin YS, Grados M, Fyer AJ, Rauch S, et al. Evidence for potential relationship between SLC1A1 and a putative genetic linkage region on chromosome 14q to obsessive-compulsive disorder with compulsive hoarding. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147:1000–2. doi: 10.1002/ajmg.b.30713. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Leckman JF, Pauls DL, Tsai CP, Kidd KK, Rosario Campos M. Genomewide Scan of Hoarding in Sib Pairs in Which Both Sibs Have Gilles de la Tourette Syndrome. Am J Hum Genet. 2002;70:896. doi: 10.1086/339520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alemany-Navarro M, Cruz R, Real E, Segalàs C, Bertolín S, Rabionet R, et al. Looking into the genetic bases of OCD dimensions: a pilot genome-wide association study. Transl Psychiatry. 2020;10. 10.1038/S41398-020-0804-Z. [DOI] [PMC free article] [PubMed]
- 35.Perroud N, Guipponi M, Pertusa A, Fullana MA, Iervolino AC, Cherkas L, et al. Genome-wide association study of hoarding traits. Am J Med Genet B Neuropsychiatr Genet. 2011;156:240–2. doi: 10.1002/ajmg.b.31152. [DOI] [PubMed] [Google Scholar]
- 36.Tolin DF, Frost RO, Steketee G. A brief interview for assessing compulsive hoarding: the Hoarding Rating Scale-Interview. Psychiatry Res. 2010;178:147. doi: 10.1016/j.psychres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagai U, Lichtenstein P, Pedersen NL, Magnusson PKE. The Swedish Twin Registry: Content and Management as a Research Infrastructure. Twin Res Hum Genet. 2019;22:672–80. doi: 10.1017/thg.2019.99. [DOI] [PubMed] [Google Scholar]
- 38.Anckarsäter H, Lundström S, Kollberg L, Kerekes N, Palm C, Carlström E, et al. The Child and Adolescent Twin Study in Sweden (CATSS) Twin Res Hum Genet. 2011;14:495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 39.Magnusson PKE, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, et al. The swedish twin registry: establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16:317–29. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- 40.Boomsma DI, Vink JM, Beijsterveldt TCEM, van, Geus EJC, de, Beem AL, Mulder EJCM, et al. Netherlands twin register: a focus on longitudinal research. Twin Res Hum Genet. 2002;5:401–6. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- 41.Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, et al. Response inhibition and ADHD traits: correlates and heritability in a community sample. J Abnorm Child Psychol. 2013;41:497. doi: 10.1007/s10802-012-9693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, et al. A diffusion tensor imaging studyin children with ADHD, autism spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry. 2016;173:1213–22. doi: 10.1176/appi.ajp.2016.15111435. [DOI] [PubMed] [Google Scholar]
- 43.Tolin DF, Frost RO, Steketee G, Fitch KE. Family burden of compulsive hoarding: Results of an internet survey. Behav Res Ther. 2008;46:334–44. doi: 10.1016/j.brat.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton CL, Lemire M, Xiao B, Corfield EC, Erdman L, Bralten J, et al. Genome-wide association study of pediatric obsessive-compulsive traits: shared genetic risk between traits and disorder. Transl Psychiatry. 2021;11:1–10.. doi: 10.1038/s41398-020-01121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park LS, Burton CL, Dupuis A, Shan J, Storch EA, Crosbie J, et al. The Toronto Obsessive-Compulsive Scale: Psychometrics of a Dimensional Measure of Obsessive-Compulsive Traits. J Am Acad Child Adolesc Psychiatry. 2016;55:310–18.e4. doi: 10.1016/j.jaac.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36.. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- 47.McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48:1279–83. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed]
- 49.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang L, Zheng Z, Qi T, Kemper KE, Wray NR, Visscher PM, et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat Genet. 2019;51:1749–55. doi: 10.1038/s41588-019-0530-8. [DOI] [PubMed] [Google Scholar]
- 51.Willer CJ, Li Y, Abecasis GR. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lam M, Awasthi S, Watson HJ, Goldstein J, Panagiotaropoulou G, Trubetskoy V, et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics. 2020;36:930–3. doi: 10.1093/bioinformatics/btz633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput Biol. 2015;11:1–19.. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe K, Taskesen E, Van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1–10.. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies, OCGAS. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2017;23:1181–8. doi: 10.1038/mp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trubetskoy V, Padiñas AF, Qi T, Pagagiotaropoulou G, Awasthi S, Bigdeli T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019;8:1–6. doi: 10.1093/gigascience/giz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–66. doi: 10.1093/biomet/37.3-4.256. [DOI] [PubMed] [Google Scholar]
- 65.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 66.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–9. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wunderle VM, Critcher R, Ashworth A, Goodfellow PN. Cloning and Characterization ofSOX5,a New Member of the HumanSOXGene Family. Genomics. 1996;36:354–8. doi: 10.1006/geno.1996.0474. [DOI] [PubMed] [Google Scholar]
- 69.Lee PH, Anttila V, Won H, Feng YCA, Rosenthal J, Zhu Z, et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–82.e11. doi: 10.1016/j.cell.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe K, Stringer S, Frei O, Umićević Mirkov M, de Leeuw C, Polderman TJC, et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet. 2019;51:1339–48. doi: 10.1038/s41588-019-0481-0. [DOI] [PubMed] [Google Scholar]
- 71.Nagel M, Jansen PR, Stringer S, Watanabe K, De Leeuw CA, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449, 484 individuals identifies novel genetic loci and pathways. 2018;50. 10.1038/s41588-018-0151-7. [DOI] [PubMed]
- 72.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–86. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49:834–41. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebejer JL, Duffy DL, Van Der Werf J, Wright MJ, Montgomery G, Gillespie NA, et al. Genome-wide association study of inattention and hyperactivity-impulsivity measured as quantitative traits. Twin Res Hum Genet. 2013;16:560–74. doi: 10.1017/thg.2013.12. [DOI] [PubMed] [Google Scholar]
- 75.Middeldorp CM, Hammerschlag AR, Ouwens KG, Groen-Blokhuis MM, St. Pourcain B, Greven CU, et al. A Genome-Wide Association Meta-Analysis of Attention-Deficit/Hyperactivity Disorder Symptoms in Population-Based Pediatric Cohorts. J Am Acad Child Adolesc Psychiatry. 2016;55:896–905.e6. doi: 10.1016/j.jaac.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frost RO, Steketee G, Williams L. Hoarding: a community health problem. Heal Soc Care Community. 2000;8:229–34. doi: 10.1046/j.1365-2524.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 79.Mathews CA, Uhm S, Chan J, Gause M, Franklin J, Plumadore J, et al. Treating Hoarding Disorder in a real-world setting: Results from the Mental Health Association of San Francisco. Psychiatry Res. 2016;237:331–8. doi: 10.1016/j.psychres.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernández de la Cruz L, Nordsletten A, Mataix-Cols D. Ethnocultural aspects of Hoarding Disorder. Curr Psychiatry Rev. 2016;9:115–23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The meta-analyzed summary statistics will be made available via the Psychiatric Genomics Consortium Download page (https://www.med.unc.edu/pgc/download-results/).