Abstract
Different from many previous theoretical studies, this paper explores the regulatory mechanism of the spike and wave discharges (SWDs) in the reticular thalamic nucleus (TRN) by a dynamic computational model. We observe that the SWDs appears in the TRN by changing the coupling weights and delays in the thalamocortical circuit. The abundant poly-spikes wave discharges is also induced when the delay increases to large enough. These discharges can be inhibited by tuning the inhibitory output from the basal ganglia to the thalamus. The mechanisms of these waves can be explained in this model together with simulation results, which are different from the mechanisms in the cortex. The TRN is an important target in treating epilepsy, and the results may be a theoretical evidence for experimental study in the future.
Keywords: Spike and wave discharges, Reticular thalamic nucleus, Computation model, Control
Introduction
The spike and wave discharges (SWDs) with 2–4 Hz is the main clinical feature on the electroencephalogram (EEG) of absence epilepsy patients (Berman et al. 2010). Many previous studies have verified that epilepsy seizures may be caused by pathological factors and abnormal interactions in the cortical-thalamic (CT) circuit (Bomben et al. 2016; Meeren et al. 2002; Assenza et al. 2020; Wendling et al. 2017; Marten et al. 2009). Thalamocortical model simulations showed that an increase in nucleus reticularis thalami (NRT) cell bursting appeared before SWDs, which in turn was mediated by T-type current, and there were multiple pathological pathways leading to SWDs (Dervinis 2016). Sorokin et al. found that toggling between phasic and tonic firing modes in thalamocortical neurons was required to induce and inhibit absence epilepsy, respectively (Sorokin et al. 2017). Dayani et al. pointed out that the connection pathways in the CT network might play a key role in the generation of idiopathic generalized epilepsy, via the magnetic resonance spectroscopy technique (Dayani et al. 2017). However, the exact origin mechanism of the seizure is not clear, which leads to difficulties in treatment. The “cortical focus” theory was based on the assumption that seizure activity firstly generated and generalized rapidly over the cortex, then spread to the thalamus, and finally was maintained by the interactions between cortex and thalamus (Meeren et al. 2002, 2005). Ernst et al. provided the first evidence that the elevation of brain T-type currents can promote the improvement of gene activity of CT network, and then leads to generating the seizure wave in cortex (Ernst et al. 2009). Recently, Currie et al. found that alterations in cortical circuitry activities were earlier than the generation of seizures by more than seven days (Currie et al. 2017). There were also many theoretical models supporting that the SWDs may origin and maintain in cortex (Marten et al. 2009; Yang and Robinson 2019; Hu et al. 2017; Deeba et al. 2019), which displayed many abundant dynamical mechanisms. Therefore, the cortex is selected as a common target in treating epilepsy (Young et al. 2018; Kurada et al. 2020; Van Heukelum et al. 2016).
In addition to the cortex, the thalamus may lead the cortex and maintain the rhythmicity of seizures (Martín-López et al. 2017). Many recent studies have shown that the deep brain stimulation (DBS) exerted in the thalamus has a better therapeutic effect (Middlebrooks et al. 2018; Kim et al. 2017; Du et al. 2021; Klinger and Mittal 2018). Van Der Vlis et al. advanced that the DBS acting on the anterior nucleus of the thalamus (ANT) was well-tolerated, safe and effective for drug-resistant patients (Van Der Vlis et al. 2019). Du et al. pointed out that ANT-DBS has protection effects on the putamen and caudate, which can obviously enhance the role of basal ganglia (BG) in regulating seizure (Du et al. 2021). Park et al. observed that ANT-DBS had positive effects on refractory epilepsy patients who experienced failed vagus nerve stimulation (Park et al. 2019). In the anatomical structure, the reticular thalamic nucleus (TRN) locates at the interface between the cortex and thalamus, and receives excitatory projections both from the cortex and thalamus in the CT network of the brain (Guillery et al. 1998). Recently, many studies indicated that the TRN might be a potential target for treating epilepsy (Pantoja-Jiménez et al. 2014; Chang et al. 2017; Magdaleno-Madrigal et al. 2019). Steriade et al. found that GABAergic reticular neurons played a key role in generating of unconsciousness states during absence epilepsy (Steriade 2005). The interactions between thalamic sub-regions were mediated by the TRN, the abnormal function of which was involved in the generation of alpha rhythms in idiopathic generalised epilepsy (Bagshaw et al. 2017). Barad found that the deficient AMPAR expression contributed to seizure generation and propagation in a mouse model (Barad 2017). TRN might be a target for controlling resistant neuropsychiatric diseases and it had positive effects (Gerardo and Manuel 2020). However, the therapeutic mechanism of the DBS on the TRN is unclear and lack of theoretical evidences. Therefore, we have reason to believe that in some cases seizures originate and manifest in the thalamus. However, there is lack of theoretical evidences to support this view, and the origin mechanism of SWDs appearing directly in the thalamus little involves. In this paper, we try to explore the onset mechanism of SWDs, which appears in the TRN, in a CT network.
The brain is a complex network with strong self-regulation ability, the mechanism of which is very important for controlling seizures. The BG locates in the deep part of the white matter of the brain, which is mainly composed by the striatum, globus pallidus (GP), substantia nigra (SN) and subthalamic nucleus (STN), which has close input and output relationships with the CT network (Graybiel 2000; Johnson 2016). Many experimental studies showed that activity states in the BG greatly affected the movement regulation function of the brain (Friend and Kravitz 2014; Ikeda et al. 2015). For example, the BG exerted an important influence on the maintenance and adjustment of epilepsy seizures (Deransart et al. 1998; Deransart and Depaulis 2002; Biraben et al. 2004; Luo et al. 2012; Bouilleret et al. 2008; Slaght et al. 2002). Based on resting-state fMRI data, Luo et al. showed that the BG network is a key modulator in generalized epilepsy (Luo et al. 2012). Chiriboga et al. observed striking basal ganglia imaging abnormalities in a faciobrachial dystonic seizure patient (Chiriboga et al. 2017). Dong et al. found the possible mechanism of frontal lobe epilepsy, which mainly involved important connections in the cortex-BG circuits (Dong et al. 2016). Vuong et al. provided the evidence to indicate the critical effects of the BG in propagation and inhibition of absence and temporal lobe epilepsy (Vuong and Devergnas 2017). The important effect of the BG in focal epilepsies was also shown by changing in the resting-state connectivity between subcortical and cortical neurons (Výtvarová et al. 2017). Recently, Ferrari et al. found that progressive involvement of striatum and pallidus neurons in the BG may be a possible predictive marker of super refractory status epilepticus (Ferrari et al. 2017). In the computational model, Chen et al. found that the slow SWDs of absence epilepsy might be adjusted via the competition mechanism between pathways from the BG to the thalamus (Chen et al. 2014) and the connection projected from globus pallidus to cortex (Chen et al. 2015, 2016). Arakaki et al. found that the striatal feedforward inhibition exerts a key influence on the maintenance of seizures (Arakaki et al. 2016). However, the control mechanism of epilepsy seizures appearing in the TRN has rarely been involved by now. In this paper, we study the regulation mechanism of the SWDs in the TRN by inhibitory projections from the BG to the CT loop.
The model and method are introduced in “The network model introduction and numerical analysis method” section; the mechanisms are given in “The onset and control mechanism of the SWDs” section; the robustness of the model is analyzed in “The effect of the parameters on inhibiting of the SWDs” section; and at last, the conclusion is drawn in “Conclusion” section.
The network model introduction and numerical analysis method
Model
In the anatomy of the brain, the striatum, STN, GP and SN are main organizations in the BG. The striatum is divided into D1 and D2 neurons due to different dopamine receptor expressions. The GP contains external segment (GPe) and internal segment (GPi). The GPi and substantia nigra pars reticulata (SNr) are both main output organizations of the BG, which were often taken as a single nucleus in previous theoretical studies (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009). Except for the excitatory projections of the STN that project to the GP and SNr, other connections in the BG are inhibited. The CT network is responsible for the origin mechanism of epileptic seizures, which has close projections with the BG, and they together constitute a BGCT circuit in the brain (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009). In all, the CT network projects excitatory outputs to the BG, and in turn, the SNr and GPi exert inhibitory outputs to the thalamus. Although the cortex exerts excitatory projections to the STN and striatum, it contains some inhibitory interneurons (IIN), which have inhibitory projections to the excitatory corticocortical neurons (ECN). Following the previous models (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009), the thalamus is divided into the TRN and the relay nuclei (SRN), both of which have dense glutamatergic projections with the cortex. The TRN projects strong inhibitory outputs to the SRN, which causes signaling hysteresis in this pathway. The neuronal nuclei in Fig. 1 are abbreviated as follows (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009): r=TRN; s=SRN; =GPe; =SNr/GPi; =STN; =striatal D1 neurons; =striatal D2 neurons; e=ECN; i=IIN. is supposed as a constant subthalamic input. Blue lines indicate excitatory connections regulated by glutamate receptors. Yellow lines indicate inhibitory connections regulated by -aminobutyric acids (GABA) receptors. The synaptic dynamics of is slower than that of , which causes a delay in the projection “ TRN SRN ”.
Fig. 1.
The BGCT mean-field model (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009). The neuronal nuclei are abbreviated as follows: r=TRN; s=SRN; =GPe; =SNr/GPi; =STN; =striatal D1 neurons; =striatal D2 neurons; e=ECN; i=IIN. Blue lines indicate excitatory connections regulated by glutamate receptors. Yellow lines indicate inhibitory connections regulated by -aminobutyric acids (GABA) receptors
The coupling relationship in Fig. 1 is described by the following mean-field equations (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009):
is the mean cell-body potential of each nerve nuclei , is the incoming pulse rates and is the average discharge rate. The correlation of them is depicted by a sigmoidal function (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009):
| 1 |
is the mean threshold potential, is a standard deviation. Note that we assume that and for similar internal connectivities between ECN and IIN (Chen et al. 2014, 2015, 2016), so, the potential and discharge rate of IIN are omitted in the equations.
The of each nucleus “a” is depicted by the following second-order differential equation (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009):
| 2 |
| 3 |
is the coupling weight from the nucleus “b” to the nucleus “a”. A is a set of nerve nuclei, which have input projections to the nucleus “a”. A delay is introduced in the projection to represent its slow synaptic dynamics, which is due to the receptor -regulated connection acts through second messenger processes (Chen et al. 2014, 2015, 2016). The delay in other pathways has been omitted in this model. and are the decay and rise rates of the . is a second-order differential operator.
At last, the propagation of the pulse field along the EPN is described by the equation (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009):
| 4 |
is the damping rate of the EPN. Note that the pulse fields of other nuclei are simplified as ) in this model (Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009).
Method and data
We use the standard fourth-order Runge-Kutta algorithm to solve the equations in the Matlab software, and analytical techniques are adopted from previous studies (Chen et al. 2014, 2015, 2016). By changing a parameter gradually, we store local maxima and minima of the to get one-dimensional bifurcation diagrams in Fig. 3. Similarly, we obtain the two-dimensional bifurcation diagrams, such as Fig. 4a1. The dominant frequency (DF) of oscillations is defined by the power spectral analysis of the . The mean discharge rates (MDs) are simulated by averaging the in a long enough time (Fig. 6i) (Table 1).
Fig. 3.
A–G The one-dimensional state bifurcation diagrams, which are obtained by changing different parameters (, , , , , and , respectively) in the corticothalamic system. is the pulse field of TRN. (1),(2),(3) and (4) represent the low firing state, simple periodic oscillation state, SWDs state and maximum firing rate state, respectively. In one cycle, the state (1) and (4) have one extreme point, the state (2) has two extreme points and the state (3) has four extreme points. We set =1.4 and =1.8 in all simulations
Fig. 4.
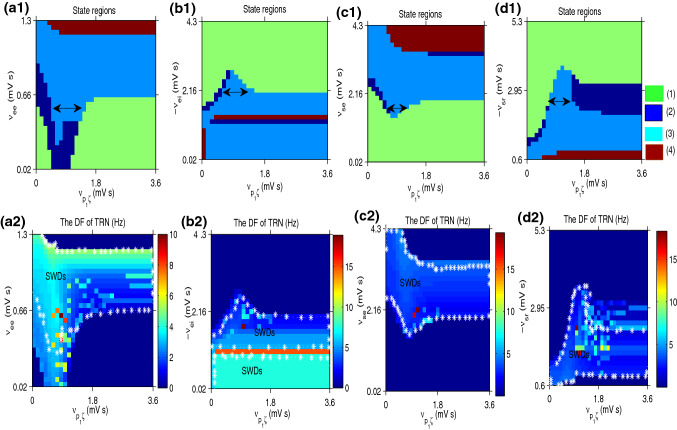
a1–d1 The state bifurcation results in (), (), () and (), respectively. is the connection strength from the STN to the SNr, which can adjust the activation level of SNr. Different oscillation states are distinguished by four different colors. They show that the SWDs may be inhibited via decreasing or increasing in , as indicated by bidirectional arrows. a2–d2 The corresponding DF calculation results, which show that the DF of SWDs state is in 2–4 Hz. In all simulations, we set =1 in (a); =1.4 in (b) and (c)
Fig. 6.
The polyspike wave discharges (PSWDs) appears in the TRN when the increases to large enough. a and b The state and DF simulation results obtained in (). Different firing states are expressed by four different colors in (a). P3 represents the polyspike wave discharges state in (a), and the 2–4 Hz PSWDs region is denoted as “PSWDs” in (b). c–h Several time series of the PSWDs, simulated by taking different values of the , which show that the number of pulses in one period increases with the increasing in delay. i The MDs of the TRN with the increasing of , obtained by setting =2 mV s. It is shown that the increase of may enhance the activation level of the TRN.
Table 1.
The default data in the simulation are derived from previous studies (Marten et al. 2009; Chen et al. 2014, 2015, 2016; Van Albada et al. 2009; Van Albada and Robinson 2009; Lytton 2008; Destexhe 1998, 1999; Robinson et al. 2002; Suffczynski et al. 2004; Breakspear et al. 2005; Da Silva et al. 2003; Taylor et al. 2013; Kuhlmann et al. 2015; Robinson et al. 2004)
| –1.8 | 0.5 | 1.8 | 0.45 | –0.075 | 2.2 | ||||||
| –0.035 | -0.035 | 0.1 | 0.7 | -0.3 | –0.1 | ||||||
| –0.03 | 0.3 | 0.05 | –0.04 | 0.05 | 1 | ||||||
| 0.1 | –0.2 | (-2)-(-0.4) | 1 | –0.3 | 250 | ||||||
| 500 | 300 | 250 | 250 | 65 | 250 | ||||||
| 65 | 250 | 100 | 100 | 50 | 200 | ||||||
| 6 | 50 | 2 | 15 | 10 | 15 | ||||||
| 15 | 10 | 19 | 19 | 9 | 15 |
The onset and control mechanism of the SWDs
From the section of introduction, we may infer that pathological factors in the CT system can induce the SWDs in the TRN. Similar to previous studies (Chen et al. 2014, 2015, 2016), Fig. 2 are four time series diagrams of the TRN, which describe different states of the model under different parameters. Fig. 2A is a low firing stable state (1). Fig. 2B is a simple oscillation state (2), which is a periodic discharge phenomenon with a pair of maximal and minimum values in one period. Fig. 2C is also a periodic firing phenomenon with two pairs of maxima and minima in a period, and it represents the SWDs state (3) in the TRN. Fig. 2D is a maximal firing stable state (4), which represents the highest activation level of the TRN. We observe that these states can be distinguished by different extreme point characteristics in a period, which will be employed to study state bifurcation processes in the following. Several state bifurcation diagrams are simulated in Fig. 3, which are obtained by changing different parameters in the CT. affects the TRN by the excitatory projections and . Therefore, exerts overall excitatory effects on the TRN, and the state (1) transfers to the state (3) with the increasing of , shown in Fig. 3(a). Similarly, we can easily analyze from Fig. 1 that and also have overall excitatory roles on the TRN; when or increases to large enough, the SWDs state appears, as shown in Fig. 3b and 3e, respectively. represents the strength of the projection from the EPN to the TRN, the increase of which will promote the activation level of the TRN directly. However, at the same time, the increase of will also result in increasing of the strength in the pathway , which in turn exerts inhibitory effects on the TRN. And, the increase of the inhibitory effect induced by is faster than the excitatory effect induced by the itself. Therefore, with the increasing of , the activation level of the TRN decreases, and the state (3), (2) and (1) appear sequentially, as shown in Fig. 3c. The effect of on the TRN is similar to that of , so, the SWDs state appears only when the is relative small, shown in Fig. 3d. It can be easily observed that and have overall inhibitory roles on the TRN, so, the SWDs state disappears with the increasing of or , shown in Fig. 3f and 3g.
Fig. 2.
The different firing states in the TRN, which are obtained by taking =0.7, =1.5, =1 and =4, respectively. is the pulse field of TRN. A A low firing stable state (1). B A simple periodic oscillation state (2). C A typical SWDs state (3), which is a periodic firing phenomenon with two pairs of maxima and minima in a period. D A maximal firing stable state (4). We set =1.4 and =1.8 in all simulations
In this model, the SNr is the main output population of the BG to the CT network, the activation level of which is greatly affected by the excitatory coupling weight . Now, we explore the regulatory mechanism of BG on SWDs activities by tuning the . Fig. 4a–d are the state and DF simulated results in the panel (), (), () and (), respectively. They are shown that SWDs may be well inhibited via both reducing or enhancing in , as indicated by the arrow. The control effects are achieved through the competition between the projections from the SNr to the thalamus and the loops in the CT. We observe in Fig. 4a1–c1 that the SWDs state and saturated state only appear when the excitatory coupling strengths and are large enough. On the contrary, the TRN mainly stays in the low firing state when the inhibitory coupling weighs and increase to large enough, as shown in Fig. 4b1 and d1. The control mechanisms are different for these different pathological factors. The DF of the SWDs in the TRN is main in 2–4 Hz.
The effect of the parameters on inhibiting of the SWDs
The coupling weight and delay are two main types of parameters considered in this model. In this section, we study the effect of these parameters on the adjustment of SWDs.
The influence of the in inhibiting of the SWDs
Figure 5 describes five groups of the state and DF bifurcation analysis in (). Here, we set to represent the competitive relationship of the two inhibitory output coupling weights and (Chen et al. 2014, 2015, 2016), KK is a constant. The states (1)-(4) are described by different colors in Fig. 5a1–e1. With the increase in , the region of SWDs states and saturated states gradually becomes larger, and the state (1) and (2) disappear gradually, so has an over excitatory effects on TRN. The DF is enhanced with the increase in , as presented in Fig. 5 a2–e2. The “bidirectional inhibition” phenomenon is shown in Fig. 5a1 as the is small and the KK is large enough. We infer that the roles of and are well-matched in strength in this case. When the increases to large enough, such as Fig. 5c1, we infer that the role of the is dominant in this case. And, the minimum value of KK is required for controlling decreases with the increasing of . On the one hand, the results in Fig. 5 show that the dynamic behaviors have good robustness within the small range of parameter changes, such as Fig. 5a–e, which indicate that the model and method are effective for describing the SWDs activities in the TRN. On the other hand, the effect of on control is obvious when it changes greatly.
Fig. 5.
The effect of the parameter on control. a1–e1 The state simulation results in (). Here, we define , and KK is a constant, which represents the competitive relationship between and . They are simulated via taking =0.8, =1, =1.5, =2 and =2.3, respectively. Different oscillation states are distinguished by four different colors, which show that the dynamic behaviors have good robustness within some small ranges of parameter changes, such as (a) and (b), or, (c), (d) and (e). And, the has great effect on control when it changes greatly. a2–e2 The corresponding DF calculation results
The effect of the on SWDs states
Previous computational model studies have reported that the delay was a key parameter in inducing different slow poly-spikes wave discharges (PSWDs) in the cortex (Marten et al. 2009; Hu et al. 2017). In this section, we explore the effect of the on SWDs states in the TRN. We find that the PSWDs appears in the TRN when the in increases to large enough. Fig. 6a and b describe the state and DF bifurcation in (). Here, the state (P3) represents the polyspike wave discharges. Fig. 6a clearly shows that the region range of the saturated state (4) becomes larger with the increasing of the delay, which implies that appropriate delays can promote the activation level of TRN. Fig. 6c–h are several time series of the PSWDs, which are simulated by taking different values of the ; they show that the number of pulses in one period increases with the increasing of the delay. Fig. 6i is the MDs of the TRN with the increasing of , obtained by setting =2 mV s, which clearly shows that the increase of may enhance the firing ability of TRN. In fact, we infer from the model that the inhibitory pathway affects the firing of TRN mainly through two pathways and . Therefore, the increase of can weaken the inhibitory effect of , which in turn strengthens the excitatory effect in TRN. Thus, the PSWDs appears in TRN when the delay increases to large enough.
Now, we employ an external stimulus voltage V in SNr to explore the control mechanism of the PSWDs. Fig. 7a and b are the state and DF bifurcation results in (V, ). The PSWDs can be inhibited by tuning V to large enough, indicated by the arrow. Fig. c and d describe the bifurcation diagrams of time series with the linear increase in V, simulated by taking =0.6 s in Fig. 7a. Therefore, Fig. 7 implies that the control strategy used in the above may be suitable for similar SWDs phenomena in the TRN.
Fig. 7.
The inhibition effect of the PSWDs by employing an external stimulus voltage V in the SNr. a–b The state and DF computational results. The PSWDs can be inhibited by tuning V to large enough, indicated by the arrow. c–d The transition process of the time series with the linear increase of the V, simulated by taking =0.6 s in (a). In all simulations, we set =1.5 mV s
Conclusion
Many previous theoretical model studies have focused on the mechanism of epileptic SWDs in the cortex. The mechanism of the thalamus that involves in inducing and controlling SWDs is unclear. In this paper, we explore the origin and adjustment mechanism of the SWDs in a dynamic calculation model. We observe that the SWDs appear in the TRN by changing different coupling weights in the CT loop. The origin mechanism can be explained in this model together with simulation results. The PSWDs can also appear in the TRN when the delay increases to large enough, which is often used to distinguish different types of absence seizure-like activities. The larger the delay is, the more the number of extreme points in one period will be. These SWDs can be inhibited by tuning the outputs from the BG to the CT network and the external stimulation in the SNr. We find the phenomenon of two-way regulation and the DF of the SWDs in the TRN is about 2–4 Hz. We should point out that the model is effective in describing SWDs, which shows good parameter robustness in Fig. 5.
The selection of effective target region in epilepsy treatment is a hot issue, and its mechanism is not clear. In this paper, we try to adjust seizure-like activities by changing the coupling strength from the STN to SNr, which can affect the activation level of SNr directly. And, we observe that the phenomenon of two-way regulation is obvious in Fig. 4. Therefore, we infer that on the one hand the SWDs can be inhibited by tuning the state of SNr; on the other hand, the SWDs can be controlled by activating or deactivating SNr under different parameters. So, we believe that the SNr may be an effective target for treating epilepsy in clinic, and we verify it theoretically by exerting an external stimulation voltage V in the SNr, such as Fig. 7. Of course, the clinical treatment mechanism of epilepsy is complex, and the specific treatment scheme may vary from person to person. We hope that the results obtained in this paper can provide a certain theoretical basis for experimental research.
Finally, we point out that the mechanisms of SWDs in different neural nuclei may be different. In Fig. 8, we simulate the synchronous resonance activities of the EPN, TRN and SRN in this model, which are obtained by setting different values of . It is shown that oscillation amplitudes in the TRN, SRN and EPN reduce gradually, which may indicate that the oscillating activity originally originates in the TRN and transfers to the SRN and EPN with the development of the disease. Therefore, epileptic waves appear in different areas may represent various mechanisms of epilepsy. Many previous experiments have shown that drug and external stimulations exerted in the TRN were very effective for relieving epilepsy seizures (Chang et al. 2017; Nanobashvili et al. 2003; Kohmann et al. 2016; Gummadavelli et al. 2015). The results focus on the TRN in this paper may provide a theoretical support for previous experimental studies.
Fig. 8.
The synchronous resonance phenomenon of EPN, TRN and SRN. a–d represent four different states obtained by setting different values of
Acknowledgements
This research was supported by the National Science Foundation of China (No. 11602092); the Natural Science Foundation of Hubei Province (No. 2018CFB628); the China Postdoctoral Science Foundation (No. 2018M632184).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bing Hu, Email: djhubingst@163.com.
Dingjiang Wang, Email: wangdingj@126.com.
References
- Arakaki T, Mahon S, Charpier S, et al. The role of striatal feedforward inhibition in the maintenance of absence seizures. J Neurosci. 2016;36(37):9618–9632. doi: 10.1523/JNEUROSCI.0208-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenza G, Lanzone J, Dubbioso R, et al. Thalamic and cortical hyperexcitability in juvenile myoclonic epilepsy. Clin Neurophysiol. 2020;131(8):2041–2046. doi: 10.1016/j.clinph.2020.04.164. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Hale JR, Campos BM, et al. Sleep onset uncovers thalamic abnormalities in patients with idiopathic generalised epilepsy. Neuro Image Clin. 2017;16:52–57. doi: 10.1016/j.nicl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad Z(2017) Excitatory ionotropic glutamate receptor expression in the reticular thalamic nucleus in a mouse model of absence epilepsy. University of Otago
- Berman R, Negishi M, Vestal M, et al. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51(10):2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biraben A, Semah F, Ribeiro MJ, et al. PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy. Neurology. 2004;63(1):73–77. doi: 10.1212/01.wnl.0000132840.40838.13. [DOI] [PubMed] [Google Scholar]
- Bomben VC, Aiba I, Qian J, et al. Isolated P/Q calcium channel deletion in layer VI corticothalamic neurons generates absence epilepsy. J Neurosci. 2016;36(2):405–418. doi: 10.1523/JNEUROSCI.2555-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Semah F, Chassoux F, et al. Basal ganglia involvement in temporal lobe epilepsy: a functional and morphologic study. Neurology. 2008;70(3):177–184. doi: 10.1212/01.wnl.0000297514.47695.48. [DOI] [PubMed] [Google Scholar]
- Breakspear M, Roberts JA, Terry JR, et al. A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex. 2005;16(9):1296–1313. doi: 10.1093/cercor/bhj072. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Chang WP, Shyu BC. Suppression of cortical seizures by optic stimulation of the reticular thalamus in PV-mhChR2-YFP BAC transgenic mice. Mol Brain. 2017;10(1):1–15. doi: 10.1186/s13041-017-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Guo D, Li M, et al. Critical roles of the direct GABAergic pallido-cortical pathway in controlling absence seizures. PLoS Comput Biol. 2015;11(10):e1004539. doi: 10.1371/journal.pcbi.1004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Guo D, Wang T, et al. Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput Biol. 2014;10(3):e1003495. doi: 10.1371/journal.pcbi.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wu S, Xia Y, et al. Adv Cogn Neurodyn. Singapore: Springer; 2016. Possible critical roles of Globus pallidus externa in controlling absence seizures; pp. 633–640. [Google Scholar]
- Chiriboga ASL, Siegel JL, Tatum WO, et al. Striking basal ganglia imaging abnormalities in LGI1 ab faciobrachial dystonic seizures. Neurol Neuroimmunol. 2017;4(3):e336. doi: 10.1212/NXI.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SP, Luz LL, Booker SA, et al. Reduced local input to fast-spiking interneurons in the somatosensory cortex in the GABAA 2 R43Q mouse model of absence epilepsy. Epilepsia. 2017;58(4):597–607. doi: 10.1111/epi.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva FL, Blanes W, Kalitzin SN, et al. Epilepsies as dynamical diseases of brain systems: basic models of the transition between normal and epileptic activity. Epilepsia. 2003;44(s12):72–83. doi: 10.1111/j.0013-9580.2003.12005.x. [DOI] [PubMed] [Google Scholar]
- Dayani MA, Daneshi A, Ahadi R, et al. Diagnostic value of magnetic resonance spectroscopy in morphometrical analysis of basal ganglia in patients with idiopathic generalized epilepsy. Res J Pharm Technol. 2017;10(8):2693–2696. [Google Scholar]
- Deeba F, Sanz-Leon P, Robinson PA. Unified dynamics of interictal events and absence seizures. Phys Rev E. 2019;100(2):022407. doi: 10.1103/PhysRevE.100.022407. [DOI] [PubMed] [Google Scholar]
- Deransart C, Depaulis A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002;4(3):61–72. [PubMed] [Google Scholar]
- Deransart C, Vercueil L, Marescaux C, et al. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 1998;32(1):213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Dervinis M (2016) Pathophysiological mechanisms of absence epilepsy: a computational modelling study. Cardiff University
- Destexhe A. Can GABAA conductances explain the fast oscillation frequency of absence seizures in rodents? Eur J Neurosci. 1999;11(6):2175–2181. doi: 10.1046/j.1460-9568.1999.00660.x. [DOI] [PubMed] [Google Scholar]
- Destexhe A. Spike-and-wave oscillations based on the properties of GABAB receptors. J Neurosci. 1998;18(21):9099–9111. doi: 10.1523/JNEUROSCI.18-21-09099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Wang P, Peng R, et al. Altered basal ganglia-cortical functional connections in frontal lobe epilepsy: A resting-state fMRI study. Epilepsy Res. 2016;128:12–20. doi: 10.1016/j.eplepsyres.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Du T, Chen Y, Shi L, et al. Deep brain stimulation of the anterior nuclei of the thalamus relieves basal ganglia dysfunction in monkeys with temporal lobe epilepsy. CNS Neurosci Ther. 2021;27(3):341–351. doi: 10.1111/cns.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst WL, Zhang Y, Yoo JW, et al. Genetic enhancement of thalamocortical network activity by elevating 1G-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29(6):1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A, Renzetti P, Serrati C, et al. Serial magnetic resonance study in super refractory status epilepticus: progressive involvement of striatum and pallidus is a possible predictive marker of negative outcome. Neurol Sci. 2017;38(8):1513–1516. doi: 10.1007/s10072-017-3007-5. [DOI] [PubMed] [Google Scholar]
- Friend DM, Kravitz AV. Working together: basal ganglia pathways in action selection. Trends Neurosci. 2014;37(6):301–303. doi: 10.1016/j.tins.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo CM, Manuel MMV. The thalamic reticular nucleus: a common nucleus of neuropsychiatric diseases and deep brain stimulation. J Clin Neurosci. 2020;73:1–7. doi: 10.1016/j.jocn.2020.01.061. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr biol. 2000;10(14):R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21(1):28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Gummadavelli A, Motelow JE, Smith N, et al. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 2015;56(1):114–124. doi: 10.1111/epi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Chen S, Chi H, et al. Controlling absence seizures by tuning activation level of the thalamus and striatum. Chaos Solitons Fractals. 2017;95:65–76. [Google Scholar]
- Ikeda H, Adachi K, Fujita S, et al. Investigating complex basal ganglia circuitry in the regulation of motor behaviour, with particular focus on orofacial movement. Behav Pharmacol. 2015;26(1–2):18–32. doi: 10.1097/FBP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Johnson K. The basal ganglia. Develop Disord Brain Psychology Press. 2016;2:1–14. [Google Scholar]
- Kim SH, Lim SC, Yang DW, et al. Thalamo-cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr Dis Treat. 2017;13:2607. doi: 10.2147/NDT.S148617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger N, Mittal S. Deep brain stimulation for seizure control in drug-resistant epilepsy. Neurosurg Focus. 2018;45(2):E4. doi: 10.3171/2018.4.FOCUS1872. [DOI] [PubMed] [Google Scholar]
- Kohmann D, Lüttjohann A, Seidenbecher T, et al. Short-term depression of gap junctional coupling in reticular thalamic neurons of absence epileptic rats. J Physiol. 2016;594(19):5695–5710. doi: 10.1113/JP271811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann L, Grayden DB, Wendling F, et al. The role of multiple-scale modelling of epilepsy in seizure forecasting. J Clin Neurophysiol. 2015;32(3):220. doi: 10.1097/WNP.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurada L, Bayat A, Joshi S, et al. Antiepileptic effects of electrical stimulation of the piriform cortex. Exp Neurol. 2020;325:113070. doi: 10.1016/j.expneurol.2019.113070. [DOI] [PubMed] [Google Scholar]
- Luo C, Li Q, Xia Y, et al. Resting state basal ganglia network in idiopathic generalized epilepsy. Human Brain Mapp. 2012;33(6):1279–1294. doi: 10.1002/hbm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton WW. Computer modelling of epilepsy. Nat Rev Neurosci. 2008;9(8):626–637. doi: 10.1038/nrn2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdaleno-Madrigal VM, ContrerasMu-rillo G, Valdés-Cruz A, et al (2019) Effects of High-and Low-Frequency Stimulation of the Thalamic Reticular Nucleus on Pentylentetrazole-Induced Seizures in Rats. Neuromodul Technol Neural Interface 22(4): 425–434 [DOI] [PubMed]
- Marten F, Rodrigues S, Benjamin O, et al. Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos Trans R Soc A. 2009;367(1891):1145–1161. doi: 10.1098/rsta.2008.0255. [DOI] [PubMed] [Google Scholar]
- Martín-López D, Jiménez-Jiménez D, Cabañés-Martínez L, et al. The role of thalamus versus cortex in epilepsy: evidence from human ictal centromedian recordings in patients assessed for deep brain stimulation. Int J Neural Syst. 2017;27(07):1750010. doi: 10.1142/S0129065717500101. [DOI] [PubMed] [Google Scholar]
- Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, et al. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22(4):1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, da Silva FL, et al. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62(3):371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Middlebrooks EH, Grewal SS, Stead M, et al. Differences in functional connectivity profiles as a predictor of response to anterior thalamic nucleus deep brain stimulation for epilepsy: a hypothesis for the mechanism of action and a potential biomarker for outcomes. Neurosurg Focus. 2018;45(2):E7. doi: 10.3171/2018.5.FOCUS18151. [DOI] [PubMed] [Google Scholar]
- Nanobashvili Z, Chachua T, Nanobashvili A, et al. Suppression of limbic motor seizures by electrical stimulation in thalamic reticular nucleus. Exp Neurol. 2003;181(2):224–230. doi: 10.1016/s0014-4886(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Pantoja-Jiménez CR, Magdaleno-Madrigal VM, Almazán-Alvarado S, et al. Anti-epileptogenic effect of high-frequency stimulation in the thalamic reticular nucleus on PTZ-induced seizures. Brain Stimul. 2014;7(4):587–594. doi: 10.1016/j.brs.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Park HR, Choi SJ, Joo EY, et al. The role of anterior thalamic deep brain stimulation as an alternative therapy in patients with previously failed vagus nerve stimulation for refractory epilepsy. Stereot Funct Neuros. 2019;97(3):176–182. doi: 10.1159/000502344. [DOI] [PubMed] [Google Scholar]
- Robinson PA, Rennie CJ, Rowe DL. Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Phys Rev E. 2002;65(4):041924. doi: 10.1103/PhysRevE.65.041924. [DOI] [PubMed] [Google Scholar]
- Robinson PA, Rennie CJ, Rowe DL, et al. Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Human Brain Mapp. 2004;23(1):53–72. doi: 10.1002/hbm.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaght SJ, Paz T, Mahon S, et al. Functional organization of the circuits connecting the cerebral cortex and the basal ganglia: implications for the role of the basal ganglia in epilepsy. Epileptic Disord. 2002;4(3):9–22. [PubMed] [Google Scholar]
- Sorokin JM, Davidson TJ, Frechette E, et al. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. 2017;93(1):194–210. doi: 10.1016/j.neuron.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28(6):317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Suffczynski P, Kalitzin S, Da Silva FHL. Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Neuroscience. 2004;126(2):467–484. doi: 10.1016/j.neuroscience.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Goodfellow M, Wang Y, et al. Towards a large-scale model of patient-specific epileptic spike-wave discharges. Biol Cybern. 2013;107(1):83–94. doi: 10.1007/s00422-012-0534-2. [DOI] [PubMed] [Google Scholar]
- Van Albada SJ, Gray RT, Drysdale PM, et al(2009) Mean-field modeling of the basal ganglia-thalamocortical system. II: dynamics of parkinsonian oscillations. J Theor Biol 257(4): 664-688 [DOI] [PubMed]
- Van Albada SJ, Robinson PA(2009) Mean-field modeling of the basal ganglia-thalamocortical system. I: firing rates in healthy and parkinsonian states. J Theor Biol 257(4): 642–663 [DOI] [PubMed]
- Van Der Vlis TAMB, Schijns OEMG, Schaper FL, et al. Deep brain stimulation of the anterior nucleus of the thalamus for drug-resistant epilepsy. Neurosurg Rev. 2019;42(2):287–296. doi: 10.1007/s10143-017-0941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heukelum S, Kelderhuis J, Janssen P, et al. Timing of high-frequency cortical stimulation in a genetic absence model. Neuroscience. 2016;324:191–201. doi: 10.1016/j.neuroscience.2016.02.070. [DOI] [PubMed] [Google Scholar]
- Vuong J, Devergnas A. The role of the basal ganglia in the control of seizure. J Neural Transm. 2017;125(3):531–545. doi: 10.1007/s00702-017-1768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Výtvarová E, Mareček R, Fousek J et al (2017) Large-scale cortico-subcortical functional networks in focal epilepsies: the role of the basal ganglia. Neuro Image Clin 14:28–36 [DOI] [PMC free article] [PubMed]
- Wendling F, Bartolomei F, Modolo J (2017) Neocortical/thalamic in silico models of seizures and epilepsy//Models of seizures and epilepsy. Academic Press, pp. 233–246
- Yang DP, Robinson PA. Unified analysis of global and focal aspects of absence epilepsy via neural field theory of the corticothalamic system. Phys Rev E. 2019;100(3):032405. doi: 10.1103/PhysRevE.100.032405. [DOI] [PubMed] [Google Scholar]
- Young JC, Vaughan DN, Paolini AG, et al. Electrical stimulation of the piriform cortex for the treatment of epilepsy: a review of the supporting evidence. Epilepsy Behav. 2018;88:152–161. doi: 10.1016/j.yebeh.2018.09.004. [DOI] [PubMed] [Google Scholar]