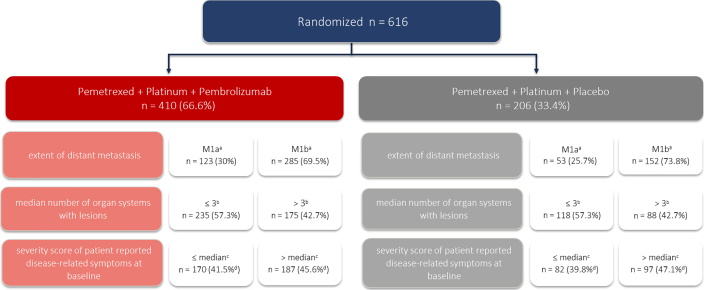
Figure 1.
Identification of the analysis population and characterization of baseline tumor burden subgroups. aThere were two patients with missing data in the treatment arm and one patient in the control arm. bMedian number of organ systems with lesions was 3. cMedian score = 122.2, on the basis of six lung-specific symptoms from the patient-reported QLQ-LC-13 instrument (coughing, dyspnea, hemoptysis, pain in arm or shoulder, pain in the chest, pain in other parts of body); the number of patients with available lung cancer-associated symptoms at baseline from the QLQ-LC13 instrument was 357 in the pemetrexed + platinum + pembrolizumab arm and 179 in the pemetrexed + platinum + placebo arm. dPercentage calculations are provided on the basis of total patient population (n = 410 and N = 206) and not total number of patients with available lung cancer-associated symptoms at baseline from the QLQ-LC13 instrument. QLC-LC13, Quality of Life Questionnaire Lung Cancer 13.