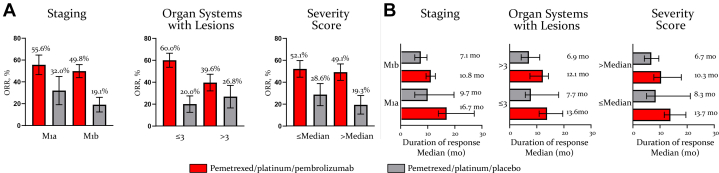
Figure 5.
ORR and DoR by baseline tumor burden. Illustrated are the ORR (A) and median DoR (B) by tumor burden subgroups. Responses are based on BICR assessment per RECIST version 1.1. In the M1a compared with M1b stage subgroups, 39.7% versus 23.3% of patients receiving pemetrexed and platinum plus pembrolizumab and 15.6% versus 6.5% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. In the subgroup with number of organ systems with lesions less than or equal to 3 compared with greater than 3, 33.7% versus 17.2% of patients receiving pemetrexed and platinum plus pembrolizumab and 0.0% versus 11.6% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. In the subgroup with symptom severity score less than or equal to the median compared with greater than the median, 27.7% versus 29.2% of patients receiving pemetrexed and platinum plus pembrolizumab and 20.2% versus 0.0% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. BICR, blinded independent central review; DoR, duration of response; ORR, objective response rate; RECIST, Response Evaluation Criteria in Solid Tumors.