Abstract

In this study, we carried out a detailed investigation of the photoluminescence of Mn4+ in Ga2O3–Al2O3 solid solutions as a function of the chemical composition, temperature, and hydrostatic pressure. For this purpose, a series of (Al1–xGax)2O3:Mn4+,Mg phosphors (x = 0, ..., 0.1.0) were synthesized and characterized for the first time. A detailed crystal structure analysis of the obtained materials was done by the powder X-ray diffraction technique. The results of the crystal structure and luminescence studies evidence the transformation of the ambient-pressure-synthesized material from the rhombohedral (α-type) to monoclinic (β-type) phase as the Ga content exceeds 15%. Spectroscopic features of the Mn4+ deep-red emission, including the temperature-dependent emission efficiency and decay time, as well as the possibility of their tuning through chemical pressure in each of these two phases were examined. Additionally, it has been shown that the application of hydrostatic pressure of ≥19 GPa allows one to obtain a corundum-like α-Ga2O3:Mn4+ phase. The luminescence properties of this material were compared with β-Ga2O3:Mn4+, which is normally synthesized at ambient pressure. Finally, we evaluated the possibility of application of the studied phosphor materials for low-temperature luminescence thermometry.
Short abstract
We carried out a detailed investigation of the photoluminescence of Mn4+ in Ga2O3−Al2O3 solid solutions as a function of the chemical composition, temperature, and hydrostatic pressure and evaluated the possibility of application of the studied phosphors for low-temperature luminescence thermometry.
Introduction
Red-emitting Mn4+ -doped compounds belong to a very promising group of phosphors with application in solid-state lighting, displays, lasers, bioimaging, and sensors.1−3 One of the first Mn4+-doped phosphors studied back in the early 1960s was the Mn4+-doped corundum (sapphire), α-Al2O3:Mn4+,4 which has been considered as an alternative of a first laser material ruby (α-Al2O3:Cr3+). Following this, many oxide compounds activated with Mn4+ ions were investigated.2 The spectroscopic properties of transition-metal ions, like Mn4+ or Cr3+ (both 3d3 configuration), are strongly influenced by the surrounding crystal field. Emission of Mn4+ ions, which usually have octahedral coordination, exhibits a sharp line due to the spin-forbidden 2E → 4A2 transition. This is due to the strong crystal field experienced typically by the Mn4+ ions because of the highly effective positive charge of the cation. The broadband emission caused by the spin-allowed 4T2 → 4A2 transition usually is not observed for Mn4+, unlike Cr3+.
Ga2O3–Al2O3 alloys (solid solutions) represent an interesting host material because their crystal structure and physical properties can be changed by modifying the composition in a wide range.5 A recently published report on the tuning of the photoluminescence properties of Cr3+ ions in the (Ga1–xAlx)2O3 host lattice gives information on the effect of Al for Ga substitution.6 In particular, it was shown that the replacement of Al by Ga in this host lattice weakens the crystal-field strength experienced by Cr3+ ions, so that the broadband emission due to the 4T2 → 4A2 transition in Cr3+ occurs at room temperature alongside the 2E → 4A2 transition. The photoluminescence properties of Cr3+ ions in β-Ga2O3 are also well-documented, and the β-Ga2O3:Cr3+ phosphor has been proposed as a promising high-pressure calibrant for a diamond anvil cell (DAC),7 for near-IR-tunable laser applications,8,9 for artificial lighting for agriculture,10 and for noncontact luminescence thermometry.11−13 The opposite effect, i.e., strengthening of the crystal field, can be achieved by applying a hydrostatic pressure, which has been demonstrated in a number of Cr3+-doped compounds.6,14−16
To the best of our knowledge, up to now, there have been no studies of Mn4+ emission in Ga2O3–Al2O3 alloys, except for pure α-Al2O3. Therefore, it is of interest to explore what may happen with the emission of Mn4+ when the α-Al2O3 crystal lattice is modified by the addition of Ga. At such a modification, one can expect the influence of at least three factors. The first is a weakening of the crystal-field strength experienced by Mn4+ ions in the corundum structure modified by gallium. The second is a decrease of the band gap of the host lattice, which will occur as the Ga content increases.5 Finally, the corundum structure of the host should switch to the monoclinic β-Ga2O3-type structure at about 20% Ga.5,6 Because of the increase of the Al(Ga)–O distances, structure alteration can cause a weakening of the crystal-field strength to the extent that the 4T2 → 4A2 emission may become possible even for Mn4+ ions. It would be also interesting to verify the degree of covalency for Mn4+–O2– bonding in Ga2O3–Al2O3 alloys using as a criterion the position of the spin-forbidden 2E → 4A2 emission line.17,18
Aiming to gain insight into these issues, we synthesized a series of original microcrystalline phosphor materials with nominal composition (Al1–xGax)2O3:Mn(0.05 atom %),Mg (0.05 atom %) with x = 0, ..., 1.0 and investigated their crystal structure and the photoluminescence properties of Mn4+ ions in the temperature range from 4.4 to 500 K. The studies are complemented by the measurements of the luminescence properties of β-Ga2O3:Mn4+ at hydrostatic pressures of up to 30 GPa.
Experimental Methods
A series of Mn- and Mg-codoped aluminum–gallium oxides of nominal composition (Al1–xGax)2O3:Mn(0.05 atom %),Mg(0.05 atom %) with x = 0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.50. 0.75, and 1.0 were produced by a sol–gel citrate method from Al(NO3)3·9H2O, Mn(NO3)2·4H2O, and Mg(NO3)2·6H2O as the initial reagents. The metallic Ga dissolved in HNO3 was used as a Ga source. Appropriate aliquots of metal nitrate solutions corresponding to a nominal composition of a sample were mixed in a magnetic stirrer for 30 min. After that, a water solution of citric acid was added to the reaction mixture, ensuring that the molar ratio of metals to citric acid was equal to 1:2. The prepared solutions were evaporated at a temperature of 353 K and dried at 373 K to form a polymer gel. Heat treatment of the obtained product was carried out in several stages: at temperatures of 573 and 725 K for 30 min to remove the organic component and subsequently at 973, 1173, and 1473 K for 4–7 h to ensure crystallization and formation of the desired phase. A final heat treatment of the product was performed at 1773 K for 7 h.
X-ray diffraction (XRD) characterization of the synthesized materials was performed with an Aeris benchtop powder diffractometer (Malvern Panalytical) equipped with a PIXcel1D strip detector. Some measurements were made using a high-resolution X’Pert MRD diffractometer (Philips). Experimental diffraction data were collected using filtered Cu Kα radiation (λ = 1.54185 Å) or a monochromated Cu Kα1 beam (λ = 1.54056 Å) in a 2θ range of 10–105° with a 2θ step of 0.01°. Crystal structure parameters (unit cell dimensions, coordinates, and displacement parameters of atoms) were derived from the experimental XRD patterns by full profile Rietveld refinement using the WinCSD software package.19
The photoluminescence (PL) and photoluminescence excitation (PLE) spectra were measured using a Horiba/Jobin-Yvon Fluorolog-3 spectrofluorometer with a 450 W continuous-wave xenon lamp for excitation. The emission was detected by a Hamamatsu R928P photomultiplier operating in a photon-counting mode. The PL spectra were corrected for the spectral response of the used system. The luminescence decay kinetics were measured using the same Fluorolog-3 spectrofluorometer, with the excitation light modulated by a mechanical chopper. Spectroscopic measurements in the temperature range 4.4–330 K were carried out on a Janis continuous-flow liquid-helium cryostat using a Lake Shore 331 temperature controller. Studies in the range 295–500 K were done in air using a compact resistive heater and an Eurotherm 902 temperature controller. The photoluminescence quantum efficiency (QE) was estimated as the ratio of the number of emitted photons to that of absorbed photons, similar to that described in ref (20).
The high-pressure luminescence measurements were carried out on a miniature DAC from easyLab placed in a CF 200 Oxford Instruments continuous-flow cryostat with an ITC4 Oxford Instruments temperature controller. The studied powder sample was ground in a mortar before loading into the cell in order to have a finer powder. Because of the fact that the studied β-Ga2O3:Mn sample contained Cr3+ ions as unintentional dopant, it was decided not to introduce a ruby as a pressure sensor but to use the R1-line position of Cr3+ in β-Ga2O3 for pressure calibration, as reported in ref (7). A methanol–ethanol mixture in a volume ratio of 5:1 was used as the pressure-transmitting medium. The luminescence was collected in a backscattering geometry using a Yobin Yvon-Spex Triax 320 monochromator equipped with a Spectrum One liquid-nitrogen-cooled CCD camera. In this experiment, the luminescence was excited by a 405 nm emission from 100 mW diode laser or by a 325 nm line from a 20 mW He–Cd laser.
High-energy-resolution fluorescence-detected X-ray absorption near-edge structure (HERFD-XANES) spectra were collected using a 1m X-ray emission Johann spectrometer at the Diamond Light Source on the I20 scanning beamline.21 For this experiment, three Ge(333) analyzer crystals were used to monitor the intensity of the Mn Ka emission line as a function of the incident energy. The spectrometer and monochromator were calibrated by measuring the Ka-line (5898.8 eV) and K-edge (6539 eV), respectively, from a Mn foil, and an elastic measurement confirmed that energy resolution of the measurements overcomes the core–hole lifetime broadening.
Results and Discussion
Phase Composition and Crystal Structure Parameters
Examination of the XRD patterns for a series of (Al1–xGax)2O3:Mn,Mg materials heat-treated at 1473 K reveled the formation of a corundum-type α-Al2O3 structure as the main phase for the Al-rich specimens with x ≤ 0.15 and a pure monoclinic θ-Al2O3 (β-Ga2O3) phase for the Ga-rich samples with x ≥ 0.5. For the intermediate compositions with x = 0.02 and 0.25, a metastable δ-Al2O3 polymorph was identified as the main phase. The phase relationships in the (Al1–xGax)2O3 system changed considerably after the final heat treatment of the samples at 1773 K for 7 h. Pure corundum and β-Ga2O3-type structures were found in the samples with x ≤ 0.05 and x ≥ 0.2, respectively, whereas the samples with intermediate compositions (x = 0.10 and 0.15) consisted of a mixture of these two phases in different proportions (Figure 1a). Thus, it was confirmed that, in the (Al1–xGax)2O3 system, the morphotropic phase transition between the rhombohedral corundum and monoclinic β-Ga2O3 types of structures occurs at 0.05 < x < 0.20 (Figure 1b). These materials were taken for further structural and luminescent investigations.
Figure 1.
XRD patterns (a) and phase compositions of the (Al1–xGax)2O3:Mn,Mg powders (b) illustrating phase evolution in the (Al1–xGax)2O3 series versus Ga content. The phase composition for 10 and 15% Ga samples was derived by quantitative full profile Rietveld analysis.
The phase compositions and crystal structures of the investigated materials were confirmed by full profile Rietveld refinement, performed in space groups R3c and C2/m for the corresponding samples. In all cases, an excellent agreement between the experimental and calculated XRD profiles was achieved (see the examples in Figure 2). The refined structural parameters for selected representatives of the α-Al2O3 and β-Ga2O3 types of structures in the (Al1–xGax)2O3:Mn,Mg series are summarized in Table 1.
Figure 2.

Graphical results of Rietveld refinement of (Al1–xGax)2O3:Mn,Mg structures with x = 0.05, 0.10, 0.25, and 0.75 (from top to bottom). The experimental XRD patterns (small black circles) are shown in comparison with the calculated patterns for the α-Al2O3 and β-Ga2O3 types of structures (blue and red lines, respectively).
Table 1. Lattice Parameters, Coordinates, and Displacement Parameters of Atoms in the Rhombohedral and Monoclinic Structures of Selected (Al1–xGax)2O3:Mn,Mg (x = 0.05, 0.10, 0.25, and 0.75) Samples.
| lattice param | atoms, sites | x/a | y/b | z/c | Biso/eq, Å2 | occupancy |
|---|---|---|---|---|---|---|
| (Al0.95Ga0.05)2O3:Mn,Mg (R3c); RI = 0.026, RP = 0.049 | ||||||
| a = 4.76550(6) Å | Al, 12c | 0 | 0 | 0.35218(8) | 1.79(3) | 0.953(4) Al3+ + 0.047(4) Ga3+ |
| c = 13.0062(2) Å | O, 18e | 0.3049(4) | 0 | 1/4 | 1.79(5) | O2– |
| (Al0.90Ga0.10)2O3:Mn,Mg (R3c,72.8 wt.%); RI = 0.034, RP = 0.111 | ||||||
| a = 4.78804(9) Å | Al, 12c | 0 | 0 | 0.35296(7) | 1.36(3) | 0.895(2) Al3+0.105(2) Ga3+ |
| c = 13.0511(3) Å | O, 18e | 0.3073(4) | 0 | 1/4 | 2.02(5) | O2– |
| (Al0.90Ga0.10)2O3:Mn,Mg (C2/m, 27.2 wt %); RI = 0.063, RP = 0.111 | ||||||
| a = 11.8816(3) Å | Al1, 4i | 0.0912(4) | 0 | 0.8015(10) | 0.59(9) | 0.890(7) Al3+ + 0.110(7) Ga3+ |
| b = 2.94055(9) Å | Al2, 4i | 0.3412(3) | 0 | 0.6826(9) | 1.51(13) | 0.897(8) Al3+ + 0.103(8) Ga3+ |
| c = 5.6685(2) Å | O1, 4i | 0.1604(7) | 0 | 0.102(2) | 2.4(2) | O2– |
| β = 104.080(2)° | O2, 4i | 0.4913(6) | 0 | 0.2517(14) | 3.2(3) | O2– |
| O3, 4i | 0.8256(6) | 0 | 0.414(2) | 2.7(2) | O2– | |
| (Al0.75Ga0.25)2O3:Mn,Mg (C2/m); RI = 0.029, RP = 0.078 | ||||||
| a = 11.9040(2) Å | Al1, 4i | 0.0911(2) | 0 | 0.7957(3) | 1.45(3) | 0.641(9) Al3+ + 0.359(9) Ga3+ |
| b = 2.94661(4) Å | Al2, 4i | 0.3423(1) | 0 | 0.6838(3) | 1.24(4) | 0.842(8) Al3+ + 0.158(8) Ga3+ |
| c = 5.67849(6) Å | O1, 4i | 0.1593(3) | 0 | 0.1106(7) | 2.23(9) | O2– |
| β = 104.080(1)° | O2, 4i | 0.4952(3) | 0 | 0.2568(5) | 2.39(10) | O2– |
| O3, 4i | 0.8280(3) | 0 | 0.4351(7) | 1.86(8) | O2– | |
| (Al0.25Ga0.75)2O3:Mn,Mg (C2/m); RI = 0.016, RP = 0.056 | ||||||
| a = 12.11953(6) Å | Al1, 4i | 0.09053(5) | 0 | 0.7947(1) | 0.72(1) | 0.18(1) Al3+ + 0.82(1) Ga3+ |
| b = 3.01145(2) Å | Al2, 4i | 0.34162(5) | 0 | 0.6862(1) | 0.70(2) | 0.32(1) Al3+ + 0.68(1) Ga3+ |
| c = 5.77046(3) Å | O1, 4i | 0.1632(2) | 0 | 0.1064(5) | 1.20(5) | O2– |
| β = 103.963(1)° | O2, 4i | 0.4962(2) | 0 | 0.2560(4) | 1.23(6) | O2– |
| O3, 4i | 0.8284(2) | 0 | 0.4353(4) | 0.95(5) | O2– | |
The lattice parameters and unit cell volumes of rhombohedral and monoclinic modifications of the (Al1–xGax)2O3:Mn,Mg structure increase systematically with increasing Ga content in the corresponding series (Figure 3). The structural parameters of the Mn/Mg-doped (Al1–xGax)2O3 solid solution studied agree well with the reference data for the end members of the system, i.e., α-Al2O3 and β-Ga2O3 and their metastable polymorphs α-Ga2O3 and θ-Al2O3, which are collected in Pearson’s crystal structure database,22 as well as with the recently reported data for Cr3+-doped (Al1–xGax)2O36 (see the left and right panes of Figure 3, respectively).
Figure 3.
Concentration dependence of the lattice parameters and unit cell volumes of the rhombohedral (left) and monoclinic (right) phases in the (Al1–xGax)2O3:Mn,Mg series (solid symbols) in comparison with the literature data for the metastable α-Ga2O3, θ-Al2O322 and Cr3+-doped (Al1–xGax)2O36 (open symbols). Hatched areas indicate a two-phase region of the system.
The average interatomic distances inside (Al/Ga)O6 octahedra and (Al/Ga)O4 tetrahedra in rhombohedral and monoclinic (Al1–xGax)2O3 are shown in Figure 4. As can be seen from the figure, the interatomic distances inside (Al/Ga)O6 octahedra and (Al/Ga)O4 tetrahedra also increase in the (Al1–xGax)2O3:Mn,Mg series, in both the rhombohedral and monoclinic structures. Note that the average intraoctahetral Ga–O distances in the monoclinic β-Ga2O3 structure are considerably larger than those in the metastable corundum-type α-Ga2O3 structure.
Figure 4.
Average interatomic distances inside (Al/Ga)O6 octahedra and (Al/Ga)O4 tetrahedra in rhombohedral and monoclinic (Al1–xGax)2O3:Mn,Mg structures. The corresponding intraoctahedral distances in the metastable α-Ga2O3 structure22 are shown as open blue circles for comparison. The hatched area indicates a two-phase region of the system.
PL and PLE of Mn4+ in Ga2O3–Al2O3 Alloys
Figure 5 demonstrates the low-temperature Mn4+ emission spectra observed for different compositions of (Al1–xGax)2O3:Mn,Mg. Inspection of the spectra reveals three different types of Mn4+ centers in the studied materials depending on their chemical composition. The first one, marked as Mn(α), corresponds to the Mn4+ ions in the corundum-type structure of the host lattice. A zero-phonon-line (ZPL) emission of this center for x = 0 peaks at 676.3 nm, which coincides with the known Mn4+ emission in α-Al2O3.4,23 This type of Mn4+ center is observed in the studied compounds, where with a corundum phase exists (x = 0, ..., 0.15; see the previous chapter). For the x = 0.1 and 0.15 compositions, besides Mn(α), another type of Mn4+ center, denoted as MnI(β), also starts to appear. The sole emission of the MnI(β) center is observed in the single-phase monoclinic compounds with x = 0.2, ..., 0.75. Admittedly, the MnI(β) center is generically associated with the monoclinic-type structure of the (Al1–xGax)2O3 host lattice. The ZPL emission peak for MnI(β) slightly shifts from 651.0 to 659.9 nm as the host composition is changed from x = 0.15 to 0.75. Judging from the position of the ZPL of MnI(β) with respect to Mn(α), one can conclude that the Mn–O bonds in monoclinic-type solid solutions have significantly lower covalency in comparison with the corundum-type (Al1–xGax)2O3. At the same time, the covalency of the Mn–ligand bonds in the monoclinic host lattice slightly increases as the Ga content increases. It should also be noted that the emission efficiency of the MnI(β) center is much lower than that of Mn(α), and it gradually decreases as the Ga content increases (Table 2). Therefore, the emission intensity of MnI(β) in the double-phase samples with x = 0.1 and 0.15 is much less than the Mn(α) intensity despite the fact that the amounts of the monoclinic phase in these samples are 27% and 70%, respectively.
Figure 5.
Normalized low-temperature PL spectra of Mn4+ ions in the (Al1–xGax)2O3 solid solutions (x = 0, ..., 1.0) calcined at 1773 K.
Table 2. Internal Quantum Efficiency of the (Al1–xGax)2O3:Mn4+ Samples (Calcined at 1773 K) Measured at 320 nm Excitation at Room Temperature.
| sample composition (x value) | QE, % |
|---|---|
| 0 | 22.6 ± 1.0 |
| 0.05 | 20.6 ± 0.5 |
| 0.1 | 16.1 ± 1.0 |
| 0.15 | 18.2 ± 1.0 |
| ≥0.2 | low, not measured |
Interestingly, a completely different Mn4+ center (λZPL = 709.7 nm at T = 4.4 K), marked as MnII(β), was found in β-Ga2O3 (x = 1.0), which has the same type of monoclinic structure. It should be stressed that no emission of the MnII(β) center was observed in any of the solid solutions with x from 0 to 0.75. At the same time, no traces of the MnI(β) center emission were found in β-Ga2O3 (x = 1.0). The fact that the MnII(β) center is revealed only in pure β-Ga2O3 indicates that this center may represent Mn4+ ions occupying Ga sites. In contrast, in monoclinic β-(Al1–xGax)2O3, the emission of the MnI(β) center is observed over a broad range of x, indicating that the nearest environment of Mn4+ is only mildly affected by Ga for Al substitution. We anticipate that the random presence of smaller Al3+ ions in a second coordination sphere allows one to accommodate the distortion in the nearest environment of Mn4+ caused by the change of the Mn–ligand distances. This assumption is based on the similarity of the ionic radii of the Mn4+ and Al3+ ions. The question remains whether the octahedral or tetrahedral Ga site is occupied by Mn4+ ions in pure β-Ga2O3.
Characteristic PLE spectra of the observed Mn(α), MnI(β), and MnII(β) centers are shown in Figure 6. The plots in the figure show that, within each structural type (α and β), the charge-transfer (CT) (∼320 nm) and 4A2 → 4T2 (∼470 nm) excitation bands slightly shift toward lower energies with increasing Ga content. This means that the crystal-field strength for Mn4+ ions decreases as the Ga content rises in both the corundum and monoclinic types of structures, which is expected in view of the increasing average interatomic Al(Ga)–O distances in the octahedra (Figure 4). The excitation spectrum of the MnII(β) center in β-Ga2O3 stands alone from the mixed compositions. Here the strong excitation band (most probably the O–Mn4+ CT band) is significantly red-shifted, exhibiting a peak at about 380 nm, while the lowest-energy excitation band centered at 540 nm is likely due to 4A2 → 4T2 transitions. It should be noted that, because of the relatively low emission intensity of the MnI(β) and MnII(β) centers, the luminescence of the unintentional Cr3+ impurity also becomes visible in the compounds with higher Ga content. In particular, Cr3+ in the studied β-Ga2O3 (x = 1.0) sample is manifested through the 4A2 → 4T1 (∼435 nm) and 4A2 → 4T2 (∼600 nm) excitation bands (Figure 6), as well as the ZPL (2E → 4A2) emission at ∼696 nm (Figure 5).11,12 As can be seen in Figure 7, the ZPL of the Mn(α) center exhibits splitting (R1 and R2 lines) of ca. 10 meV. For the MnI(β) center, no splitting can be seen in the luminescence spectra because of a larger width of the ZPL (fwhm ∼15 meV). Nonetheless, it is not unreasonable to assume a few millielectronvolts splitting of this state because of distortion of the local environment of Mn4+. This splitting is essential to explain the temperature changes of the decay time constant (see below), and, therefore, we anticipated that it is equal to 2 meV. The ZPL of the MnII(β) center is narrower (fwhm ∼6 meV) without any hint of splitting.
Figure 6.

Characteristic low-temperature PLE spectra of the studied (Al1–xGax)2O3:Mn solid solutions calcined at 1773 K.
Figure 7.
Temperature dependence of the PL spectra of different Mn4+ centers measured for (Al1–xGax)2O3:Mn with x = 0.05, 0.2, and 1.0.
The MnII(β) emission needs to be considered in more detail. The PL spectrum of this emission consists of a sharp line with some vibrionic sidebands superimposed on a broad band stretching of up to 850 nm (Figure 5), allowing one to assume that Mn4+ ions here are in a significantly weaker (intermediate) crystal field, so that a superposition of the emissions from the 2E and 4T2 states can occur. As can be seen from Figure 7, the intensity of the broad band around 740 nm with respect to the sharp line at 709 nm increases as the temperature increases even though the overall emission intensity is quickly quenched at T ≥ 100 K. Such a temperature evolution of the PL spectrum shape is inherent in the case of superposition of the emissions from the 2E and 4T2 states; however, it is also possible for the phonon-assisted 2E → 4A2 emission only.2,3
It is unusual that the ZPL emission of MnII(β) (709.7 nm at T = 4.4 K) is red-shifted with respect to the Cr3+ ZPL (∼696 nm) in the same β-Ga2O3 (x = 1.0) host. This can be explained assuming that the MnII(β) center corresponds to Mn4+ ions in the tetrahedral Ga sites of β-Ga2O3, unlike Cr3+ occupying octahedral sites in β-Ga2O3. It should be noted that the Mn4+ ions in tetrahedral coordination were already reported in the natural mineral cordierite.24 A higher covalency of the MnII(β) center, even higher than that of the octahedral Mn(α) or Cr(β) centers, can also favor the tetrahedrally coordinated Mn4+ ions in β-Ga2O3. If we assume that the MnII(β) center corresponds to Mn4+ ions in the tetrahedral Ga sites of β-Ga2O3, the ZPL emission at 709.7 nm should be caused by the spin-forbidden 2E → 4T1 transition, which corresponds to the 3d7 electron configuration in the octahedral crystal field.
Pressure-Dependent PL of Mn4+ in β-Ga2O3
To clarify the nature of the MnII(β) center in β-Ga2O3, we measured the pressure-dependent PL spectra for this sample. The results of this experiment are shown in Figure 8. Here we used 405 nm excitation to strengthen the R lines from Cr3+ observed at 695.6 and 688.9 nm at T = 85 K and ambient pressure, which was used for pressure monitoring. As one can see from the figure, the Mn-related sharp line at about 709 nm decreases in intensity relative to the broadband emission around 740 nm and merges into one structureless band as the pressure increases. This testifies unambiguously that the MnII(β) center emission in β-Ga2O3 is caused by the spin-forbidden transition from the 2E level, whereas the broad band around 740 nm with some fine structure at ambient pressure is caused by phonon-assisted components of the same transition. It should be mentioned that a similar shape of the Mn4+ emission spectrum with a narrow ZPL peak and a broad band with weakly expressed thin structure from the Stokes side of ZPL has been also observed in Sr4Al14O25:Mn4+.25
Figure 8.
Pressure dependence of the PL spectra of β-Ga2O3:Mn measured at 405 nm excitation at T = 85 K.
As can be seen in Figure 8, at pressures starting at about 10 GPa, new Cr- and Mn-related lines appear simultaneously with the disappearance of the corresponding lines in β-Ga2O3. This is related evidently with the pressure-induced phase transformation of the monoclinic β-Ga2O3 to the rhombohedral α-Ga2O3.26,27 The transformation is almost completed at pressures of about 19 GPa, which is in agreement also with the results reported for Ga2O3:Cr.6 After pressure release, the spectrum shape does not return to the previous one for β-Ga2O3, which agrees with the irreversible pressure-induced transformation from the monoclinic to rhombohedral phase.26 Consequently, the application of hydrostatic pressure in a DAC of ≥19 GPa allowed us to obtain the corundum-like α-Ga2O3:Mn, which can be synthesized only at high pressures.28 It is of interest to note that alloying of β-Ga2O3 with Al exerts a chemical pressure that decreases the external physical pressure required for the b®a structure transformation, so when the Al content exceeds 80%, ambient pressure is sufficient to obtain the high-density corundum-like structure.
The pressure-induced shift of the ZPL position for the Mn4+ and Cr3+ centers in β-Ga2O3 is shown in Figure 9. The corresponding pressure coefficients are summarized in Table 3 in comparison with other Mn4+-doped oxide materials. As one can see from the table, the pressure coefficient for Mn4+ in β-Ga2O3 does not exceed those for other materials, where Mn4+ ions are in octahedral coordination, which rules out possible tetrahedral coordination of the Mn4+ centers in β-Ga2O3. As a reminder, in the case of tetrahedral coordination of the Mn4+ centers (which corresponds to the 3d7 electron configuration in the octahedral crystal field), the emitting 2E level should move down much more rapidly as the crystal field (pressure) increases. The characteristic ZPL emission spectra of the Mn4+ and Cr3+ centers in the pressure-created α-Ga2O3 in comparison with the starting β-Ga2O3 are shown in Figure 10. Here and after the Mn4+ center in α-Ga2O3 is marked as MnII(α). It should be mentioned that we were unable to determine exactly the pressure coefficient for Mn4+ in α-Ga2O3 because at pressures above 15 GPa the R lines of Cr3+ in β-Ga2O3 used for pressure calibration disappeared almost completely (Figure 9). Therefore, the pressure values after the phase transition (P ≥ 15 GPa) were estimated only roughly based on the external force applied to the diamonds.
Figure 9.
Shift of the ZPL position with hydrostatic pressure for the Mn4+ and Cr3+ centers in β-Ga2O3 measured at T = 85 K (solid symbols) with the corresponding linear approximation (solid lines) in eV and GPa units. Open symbols represent the corresponding ZPL positions in α-Ga2O3 at ambient pressure.
Table 3. Pressure Coefficients of the Mn4+ and Cr3+ ZPL (2E → 4A2) Energies in Some Oxide Phosphors.
| material | activator | ΔE/ΔP, meV/GPa | T, K | ref |
|---|---|---|---|---|
| α-Al2O3 | Mn4+ | –1.05 (R1) | 300 | (29) |
| –1.06 (R2) | ||||
| YAlO3 (YAP) | –1.43 | 10 | (30) | |
| Gd3Ga5O12 (GGG) | –2.32 (site 1) | 15 | (31) | |
| –1.85 (site 2) | ||||
| Sr4Al14O25 | –2.64 | 300 | (25) | |
| Mg2TiO4 | –2.4 | 50 | (32) | |
| β-Ga2O3 | –2.0 | 85 | this work | |
| α-Ga2O3 | not estimated | 85 | this work | |
| β-Ga2O3 | Cr3+ | –2.4 (R1) | 85 | (7) |
| –0.6 (R2) | ||||
| –2.4 (R1) | 85 | this work | ||
| –1.2 (R2) | ||||
| β-Ga1.58Al0.4O3 | –1.5 (R1) | 300 | (5) | |
| –1.0 (R2) | ||||
| β-Ga0.38Al1.6O3 | –1.28 (R1) | 300 | (6) | |
| –1.1 (R2) | ||||
| α-Al2O3 | –0.9 (R1) | 300 | (33) | |
| –1.0 (R2) | ||||
| –0.9 (R1) | 300 | (34) | ||
| α-Ga1.58Al0.4O3 | –1.09 | 300 | (6) | |
| α-Ga0.38Al1.6O3 | –1.13 | 300 | (6) |
Figure 10.
ZPL emission of Mn4+ and Cr3+ ions in β-Ga2O3 and α-Ga2O3 at T = 4.4 K at ambient pressure.
Aiming to get an additional clue regarding the surroundings of the Mn4+ centers in β-Ga2O3, we turned our attention to X-ray absorption spectroscopy, which can be used as a sensitive local probe of the electronic structure of transition-metal ions.35 The HERFD-XANES spectra were measured from the solid solutions (Al1–xGax)2O3:Mn,Mg (x = 0.2–1), which exhibit the same monoclinic crystal structure, by monitoring the intensity of the Kα-line across the K-edge (1s→) of Mn. The measured spectra are displayed in Figure 11. The spectra show a preedge peak at 6540 eV due to the promotion of 1s electron to 3d orbital. The edge and near-edge structures are due to transitions of the core electron (1s) to higher unoccupied states and continuum of the conduction band. A comparison of the spectra reveals that they exhibit the same preedge and near-edge structures and shapes. This provides unequivocal evidence that the local coordination geometry and valence structure of Mn centers remains the same in the alloyed compounds and pristine β-Ga2O3.
Figure 11.
HERFD-XANES spectra of (Al1–xGax)2O3:Mn,Mg (x = 0.2, 0.5 and 1) at the Mn K-edge.
Temperature Dependence of the PL Efficiency
Temperature dependences of the Mn4+ emission intensity in the studied solid solutions are shown in Figure 12. Experimental data of the temperature dependence of the emission intensity (efficiency) are usually fitted by the equation
| 1 |
where I(0) is the emission intensity at T = 0 K, ΔE is the activation energy of nonradiative transitions, and A is the ratio of the nonradiative transition probability to the radiative transition probability. However, it was found that the measured experimental data shown in Figure 12 cannot be adequately fitted by the model of the simple quenching process. Instead, it was shown that the temperature dependence of Mn4+ luminescence in the materials under study can be analyzed using a two-step quenching model given by following equation:36
| 2 |
where ΔE1 and ΔE2 are the activation energies of two competing nonradiative pathways with the corresponding parameters A1 and A2 related to the strength of the quenching processes. The use of eq 2 gives a better agreement of the theoretical curve with the experimental data, as shown in Figure 12. The energies ΔE1 and ΔE2 derived from the fitting are collated in Table 4. Here the uncertainties of the ΔE1 and ΔE2 energies were taken directly from the fitting as a fitting error. It should be mentioned that some of the compositions show a negative thermal quenching at low temperature (Figure 12). However, the low number of experimental points and their large spread in this low-temperature range do not allow us an accurate analysis in this temperature range using the approach proposed by Adachi.37 Therefore, thermal quenching starting only from the highest intensity (or starting from a plateau region) has been analyzed and fitted by us by eq 2. The results from Table 4 and Figure 12 show that the quenching temperature (T1/2) experiences gradual shifts toward lower temperatures as the Ga content increases. For the pressure-created α-Ga2O3:Mn4+, the quenching temperature is somewhat higher than that for β-Ga2O3:Mn4+. Both of the energies ΔE1 and ΔE2 tend to decrease with increasing Ga content. One of these energies can be interpreted as the energy distance from the minimum of the potential energy of the emitting 2E state to the level where it overlaps with the potential energy of the 4A2 ground state in the configurational coordinates. The other one can be ascribed to the energy distance from 2E to some defect states in the forbidden band gap of the host material, in line with that suggested in ref (37). A decrease of the last energy distance with an increase of the Ga content looks natural because the overall band gap of the host is strongly reduced from 8.8 eV for α-Al2O3 to about 4.8 eV for β-Ga2O3.5
Figure 12.
Normalized temperature dependencies of the Mn4+ emission intensity in the (Al1–xGax)2O3 solid solutions of different compositions and crystal structures. Solid lines represent fittings of the experimental points by eq 2 (see the text for details).
Table 4. Parameters of Fits Obtained from the Temperature Dependence of the Emission Intensity (Equation 2) for Mn4+ Emission in (Al1–xGax)2O3.
| sample composition (x value) | ΔE1, meV | ΔE2, meV | T1/2,a K |
|---|---|---|---|
| 0 | 148 ± 7 | 1029 ± 6 | 380 |
| 0.05 | 91 ± 27 | 727 ± 210 | 370 |
| 0.1 | 190 ± 15 | 666 ± 40 | 336 |
| 0.15 | 186 ± 86 | 657 ± 89 | 336 |
| 0.2 | 90 ± 10 | 416 ± 17 | 285 |
| 0.25 | 16 ± 3 | 272 ± 24 | 262 |
| 0.5 | 14 ± 11 | 285 ± 50 | 247 |
| 0.75 | 11 ± 3 | 142 ± 17 | 174 |
| 1.0 | 147 ± 14 | 114 | |
| 1.0b | 7 ± 2 | 100 ± 12 | 132 |
Temperature when the I(T) intensity is half of I(0).
α-Ga2O3:Mn4+.
Temperature Dependence of the PL Decay Time
The experimentally obtained temperature dependencies of the decay times of Mn4+ emission for the studied compounds are presented in Figure 13. The observed temperature dependencies can be explained by taking into account the main processes affecting the population of the emitting level 2E. A few early developed models allowed one to describe the major trends in the temperature dependence of the decay time of Mn4+.38−40 However, none of them were able to explain the rise of the decay time with heating observed at very low temperatures. This became possible with the introduction of a comprehensive model of the temperature dependence of the luminescence decay time in materials activated by transition metals developed recently by Mykhaylyk et al.12 The model accounts for the changes in the population of the emitting states due to the combined effect of thermally induced depopulation and phonon-assisted relaxation of the emission center. It should be accentuated that splitting of the 2E level included in this model is essential to explaining the initial rise of the decay time observed in the τ = f(T) curve. In this model, the radiative decay rate 1/τ(T) is derived as a weighted average of the rates from the individual levels:
 |
3 |
where Ri are the radiative decay rates, gi are the degeneracies of the states, and ΔEi is the energy difference between the ith state and the lower excited level. It has been shown that a very good quantitative interpretation of the major features of τ = f(T) characteristics of Cr3+ and Mn4+ emission can be achieved by considering the thermalization process occurring between the E̅ and 2A̅ levels, phonon-assisted relaxation and depopulation of the levels due to thermally induced transitions from 2E to an upper state that promotes further quenching of the excited states,12,23 For this group of involved levels, the expression for the decay time constant is given as follows:
 |
4 |
Figure 13.
Temperature dependencies of the decay times of Mn4+ emission in the (Al1–xGax)2O3 solid solutions of different compositions. Solid lines represent fittings of the experimental points by eq 5.
Here 1/τi (i = 1–3) are the radiative decay rates of the involved states, respectively, k is the Boltzmann constant, D is the energy split of the 2E levels, ΔE1 is the energy difference between the 2E and the upper state, and Ep stands for the “effective energy” of the phonons responsible for exchange with the sidebands.
It has been found that eq 4, however, is not adequate for fitting the temperature dependencies observed over a broader temperature range in the studied (Al1–xGax)2O3 solid solutions. The major deviation occurs in the region of thermal quenching of the emission where a rapid increase of the emission rate evidences the presence of an additional channel of nonradiative decay activated at high temperatures. It is worthwhile to note that the results of analysis of the temperature dependence of the emission intensity in materials under study give evidence of such an additional channel that requires activation energy ΔE2. We included this extra term in the model to find the relationship describing the kinetics of the system over a broad temperature range as follows:
 |
5 |
The formula was then used to fit the data obtained for the studied solid solutions. The results of the fitting shown in Figure 13 demonstrated good agreement of experiment and theory over the entire temperature range. The parameters of the fit are summarized in Table 5. It should be noted that, in the case of Ga2O3, this formula had to be modified to reflect changes in the scheme of emission transitions in the material and zero splitting for the 2E state of the Mn4+ ion.
Table 5. Parameters of Fits Obtained from the Temperature Dependence of the PL Decay Time (Equation 5) for Mn4+ Emission in (Al1–xGax)2O3.
| sample composition (x value) | τ1, ms | τ2, ms | Ep, meV | D,a meV | τ3, μs | ΔE1, meV | τ4, ms | ΔE2, meV |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.78 ± 0.02 | 1.13 ± 0.02 | 56.7 ± 1 | 10.0 | 0.1 | 225 ± 21 | 5 × 10–13 | 929 ± 46 |
| 0.05 | 0.74 ± 0.02 | 1.09 ± 0.02 | 54.4 ± 1 | 10.0 | 0.1 | 289 ± 20 | 2 × 10–11 | 784 ± 42 |
| 0.1 | 0.64 ± 0.02 | 0.80 ± 0.02 | 52.9 ± 2 | 10.0 | 0.1 | 189 ± 15 | 6 × 10–9 | 554 ± 15 |
| 0.15 | 0.64 ± 0.02 | 0.83 ± 0.02 | 56.9 ± 2 | 10.0 | 0.1 | 137 ± 5 | 2 × 10–9 | 557 ± 19 |
| 0.2 | 1.15 ± 0.01 | 1.24 ± 0.02 | 55.3 ± 2 | 2 | 0.7 | 32 ± 1 | 2 × 10–5 | 238 ± 5 |
| 0.25 | 0.98 ± 0.01 | 1.04 ± 0.02 | 50.9 ± 3 | 2 | 0.6 | 34 ± 1 | 2 × 10–5 | 245 ± 9 |
| 0.5 | 0.74 ± 0.01 | 0.79 ± 0.01 | 38.6 ± 3 | 2 | 0.57 | 35 ± 1 | 1 × 10–5 | 180 ± 20 |
| 0.75 | 0.56 ± 0.01 | 0.57 ± 0.01 | 35.0 ± 4 | 2 | 0.35 | 25 ± 2 | 4 × 10–4 | 124 ± 7 |
| 1.0 | 0.74 ± 0.01 | 27.1 ± 1 | 10 ± 4 | 1 × 10–8 | 178 ± 27 |
The value of D is fixed to be equal to the energy splitting of the 2E level (as per the spectroscopic data).
Inspection of the fit parameters reveals a gradual decrease of the values of the activation energies ΔE1 and ΔE2 correlating with the trends for the activation energies obtained from the fitting the PL intensity versus temperature plots (Figure 14). The decrease of the effective phonon energy in Ga2O3-reach samples is consistent with an increase of the mass of Ga in comparison with Al, which leads to a reduction of the vibration frequencies of the bonds. Moreover, the value of Ep for pure Ga2O3 is very close to the dominant phonon mode of 29 meV in this material.41
Figure 14.
Comparison of the activation energies ΔE1 and ΔE2 determined from eqs 2 and 5.
Thermometric Performance
The above results demonstrate efficient tuning of the temperature-dependent photoluminescence properties of the studied materials depending on the chemical composition. It is possible therefore to assume a utility of the materials for the luminescence thermometry around and below room temperature. The main interest is the temperature dependence of the decay time constant and the possibility of its tuning by changing the Al/Ga ratio in the Ga2O3–Al2O3 solid solutions.
The performance of the studied materials for thermometry
can be estimated using Figure 15, demonstrating the temperature dependencies of the
specific sensitivity 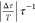 . As
one can see from the figure, the α-(Al1–xGax)2O3:Mn4+ (x = 0, ...,
0.0.1) compounds have a maximal specific sensitivity of about 5%/K
at 400 K, while the β-(Al1–xGax)2O3:Mn4+ (x = 0.2. . 0.0.5) compounds have a maximal
sensitivity of about 1%/K near room temperature. β-Ga2O3:Mn4+ discovered in this work has the highest
specific sensitivity of about 10%/K, even higher than that for β-Ga2O3:Cr3+,12 however in a narrow temperature range around 120 K.
. As
one can see from the figure, the α-(Al1–xGax)2O3:Mn4+ (x = 0, ...,
0.0.1) compounds have a maximal specific sensitivity of about 5%/K
at 400 K, while the β-(Al1–xGax)2O3:Mn4+ (x = 0.2. . 0.0.5) compounds have a maximal
sensitivity of about 1%/K near room temperature. β-Ga2O3:Mn4+ discovered in this work has the highest
specific sensitivity of about 10%/K, even higher than that for β-Ga2O3:Cr3+,12 however in a narrow temperature range around 120 K.
Figure 15.
Specific sensitivity of the decay time luminescence thermometers based on the studied Mn4+-doped Ga2O3–Al2O3 solid solutions in comparison with β-Ga2O3:Cr3+.12 The solid lines were derived from the fitting using eq 5.
Conclusions
A series of microcrystalline phosphor materials with the nominal composition (Al1–xGax)2O3:Mn(0.05 atom %),Mg(0.05 atom %) with x = 0, 0.05, 0.10, 0.15, 0.2, 0.25, 0.5, 0.75, and 1.0 were synthesized. XRD analysis of the materials calcined at 1773 K revealed pure corundum and β-Ga2O3-type structures for the samples with x ≤ 0.05 and x ≥ 0.2, respectively, whereas the samples with intermediate compositions (x = 0.10 and 0.15) consisted of a mixture of these two phases in different proportions. The crystal lattice parameters, unit cell volumes, and average interatomic distances inside (Al/Ga)O6 octahedra increase systematically with increasing of Ga content in both the rhombohedral and monoclinic modifications of (Al1–xGax)2O3 alloys.
Photoluminescence measurements of Mn4+ also confirm the appearance of the monoclinic (β-type) phase of the ambient-pressure-synthesized (Al1–xGax)2O3 solid solutions because the Ga content is more than 10%. Depending on the chemical composition and crystal phase of the host material, different types of Mn4+ centers were revealed by low-temperature photoluminescence: (i) Mn occupying most probably Al sites in α-(Al1–xGax)2O3, marked as Mn(α), (ii) Mn occupying Al octahedral sites in β-(Al1–xGax)2O3, marked as MnI(β), and (iii) Mn occupying Ga octahedral sites observed only in pure β-Ga2O3, marked as MnII(β).
It was shown that photoluminescence features of the Mn(α) and MnI(β) centers, like the temperature-dependent PL efficiency and PL decay time, can be gradually tuned in a wide range through modification of the Al/Ga ratio of the host lattice. In particular, the photoluminescence quenching temperature systematically shifts toward lower temperatures (from 380 to about 110 K) as the Ga content increases from 0 to 100%. Detailed analysis of the temperature dependencies of the PL efficiency and PL decay time allowed one to describe the main temperature-dependent processes affecting the population of the emitting level 2E.
Additionally, it was shown that the application of hydrostatic pressures of ≥19 GPa leads to the transformation of monoclinic β-Ga2O3:Mn4+ normally synthesized at ambient pressure to the rhombohedral corundum-like α-Ga2O3:Mn4+ with another emitting center, marked as MnII(α).
Finally, it was shown that the temperature range of the maximal specific sensitivity of the decay time luminescence thermometers based on the studied Mn4+-doped Ga2O3–Al2O3 solid solutions can be effectively configured below room temperature by tuning the chemical composition of the host lattice. The obtained results indicate a high application potential of the studied materials for cryogenic luminescence thermometry. In particular, the highest specific sensitivity of about 10%/K at temperatures of around 120 K was observed for β-Ga2O3:Mn4+ examined in this work.
Acknowledgments
The work was supported by the Polish National Science Centre (Projects 2018/31/B/ST8/00774 and 2021/40/Q/ST5/00336) and by the National Research Foundation of Ukraine (Grant 2020.02/0373; “Crystalline phosphors’ engineering for biomedical applications, energy saving lighting and contactless thermometry”).
The authors declare no competing financial interest.
References
- Zhou Q.; Dolgov L.; Srivastava A. M.; Zhou L.; Wang Z.; Shi J.; Dramicanin M. D.; Brik M. G.; Wu M. Mn2+ and Mn4+ Red Phosphors: Synthesis, Luminescence and Applications in WLEDs. A Review. J. Mater. Chem. C 2018, 6, 2652–2671. 10.1039/C8TC00251G. [DOI] [Google Scholar]
- Adachi S. Photoluminescence Properties of Mn4+-Activated Oxide Phosphors for Use in White-LED Applications: A review. J. Lumin. 2018, 202, 263–281. 10.1016/j.jlumin.2018.05.053. [DOI] [Google Scholar]
- Adachi S. Review - Mn4+-Activated Red and Deep Red-Emitting Phosphors. ECS J. Solid State Sci. Technol. 2020, 9, 016001. 10.1149/2.0022001JSS. [DOI] [Google Scholar]
- Geschwind S.; Kisliuk P.; Klein M. P.; Remeika J. P.; Wood D. L. Sharp-Line Fluorescence, Electron Paramagnetic Resonance, and Thermoluminescence of Mn4+ in α-Al2O3. Phys. Rev. 1962, 126, 1684–1686. 10.1103/PhysRev.126.1684. [DOI] [Google Scholar]
- Peelaers H.; Varley J. B.; Speck J. S.; Van de Walle C. G. Structural and Electronic Properties of Ga2O3-Al2O3 alloys. Appl. Phys. Lett. 2018, 112, 242101. 10.1063/1.5036991. [DOI] [Google Scholar]
- Chen K.; Fang M.; Huang W.; Kamiński M.; Majewska N.; Leśniewski T.; Mahlik S.; Leniec G.; Kaczmarek S. M.; Yang C.; Lu K.; Sheu H.; Liu R. Chemical and Mechanical Pressure-Induced Photoluminescence Tuning via Structural Evolution and Hydrostatic Pressure. Chem. Mater. 2021, 33 (10), 3832–3840. 10.1021/acs.chemmater.1c01041. [DOI] [Google Scholar]
- Beales T. P.; Goodman C. H. L.; Scarrott K. A New High Pressure Calibrant: β-Ga2O3:Cr. Solid State Commun. 1990, 73, 1–3. 10.1016/0038-1098(90)90002-S. [DOI] [Google Scholar]
- Vivien D.; Viana B.; Revcolevschi A.; Barrie J. D.; Dunn B.; Nelson P.; Stafsudd O. M. Optical Properties of β-Ga2O3:Cr3+ Single Crystals for Tunable Laser Applications. J. Lumin. 1987, 39, 29–33. 10.1016/0022-2313(87)90006-8. [DOI] [Google Scholar]
- Walsh C. G.; Donegan J. F.; Glynn T. J.; Morgan G. P.; Imbusch G. F.; Remeika J. P. Luminescence from β-Ga2O3: Cr3+,. J. Lumin. 1988, 40–41, 103–104. 10.1016/0022-2313(88)90108-1. [DOI] [Google Scholar]
- Fang M.; De Guzman G. N. A.; Bao Z.; Majewska N.; Mahlik S.; Grinberg M.; Leniec G. L.; Kaczmarek S. M.; Yang C.; Lu K.; Sheu H.; Hu S.; Liu R. Ultra-High-Efficiency Near-Infrared Ga2O3:Cr3+ Phosphor and Controlling of Phytochrome. J. Mater. Chem. C 2020, 8, 11013–11017. 10.1039/D0TC02705G. [DOI] [Google Scholar]
- Luchechko A.; Vasyltsiv V.; Zhydachevskyy Ya.; Kushlyk M.; Ubizskii S.; Suchocki A. Luminescence Spectroscopy of Cr3+ Ions in Bulk Single Crystalline β-Ga2O3. J. Phys. D: Appl. Phys. 2020, 53, 354001. 10.1088/1361-6463/ab8c7d. [DOI] [Google Scholar]
- Mykhaylyk V.; Kraus H.; Zhydachevskyy Ya.; Tsiumra V.; Luchechko A.; Wagner A.; Suchocki A. Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides. Sensors 2020, 20, 5259. 10.3390/s20185259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back M.; Ueda J.; Nambu H.; Fujita M.; Yamamoto A.; Yoshida H.; Tanaka H.; Brik M. G.; Tanabe S. Boltzmann Thermometry in Cr3+-Doped Ga2O3 Polymorphs: The Structure Matters!. Adv. Optical Mater. 2021, 9, 2100033. 10.1002/adom.202100033. [DOI] [Google Scholar]
- Kamińska A.; Suchocki A.; Kobyakov S.; Arizmendi L.; Potemski M.; Teran F. J. High-Pressure and Magneto-Optical Studies of Cr-Related Defects in the Lithium-Rich LiNbO3:Cr,Mg Crystal. Phys. Rev. B 2007, 76, 144117. 10.1103/PhysRevB.76.144117. [DOI] [Google Scholar]
- Martín-Rodríguez R.; Valiente R.; Rodríguez F.; Bettinelli M. Temperature and Pressure Dependence of the Optical Properties of Cr3+-Doped Gd3Ga5O12 Nanoparticles. Nanotechnology 2011, 22, 265707. 10.1088/0957-4484/22/26/265707. [DOI] [PubMed] [Google Scholar]
- O’Bannon E.; Williams Q. A Cr3+ Luminescence Study of Spodumene at High Pressures: Effects of Site Geometry, a Phase Transition, and a Level-Crossing. Am. Mineral. 2016, 101, 1406–1413. 10.2138/am-2016-5567. [DOI] [Google Scholar]
- Brik M. G.; Srivastava A. M. On the Optical Properties of the Mn4+ ion in Solids. J. Lumin. 2013, 133, 69–72. 10.1016/j.jlumin.2011.08.047. [DOI] [Google Scholar]
- Brik M. G.; Camardello S. J.; Srivastava A. M.; Avram N. M.; Suchocki A. Spin-Forbidden Transitions in the Spectra of Transition Metal Ions and Nephelauxetic Effect. ECS J. Solid State Sci. Technol. 2016, 5, R3067–R3077. 10.1149/2.0091601jss. [DOI] [Google Scholar]
- Akselrud L.; Grin Y. WinCSD: Software Package for Crystallographic Calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. 10.1107/S1600576714001058. [DOI] [Google Scholar]
- Zhydachevskyy Y.; Syvorotka I. I.; Tsiumra V.; Baran M.; Lipińska L.; Wierzbicka A.; Suchocki A. Quantum Efficiency of the Down-Conversion Process in Bi3+-Yb3+ and Ce3+-Yb3+ co-doped Garnets. Sol. Energy Mater. Sol. Cells. 2018, 185, 240–251. 10.1016/j.solmat.2018.05.037. [DOI] [Google Scholar]
- Hayama S.; Boada R.; Chaboy J.; Birt A.; Duller G.; Cahill L.; Freeman A.; Amboage M.; Keenan L.; Diaz-Moreno S. Photon-in/photon-out spectroscopy at the I20-scanning beamline at diamond light source. J. Phys.: Condens. Matter 2021, 33, 284003. 10.1088/1361-648X/abfe93. [DOI] [PubMed] [Google Scholar]
- Villars P.; Cenzual K.. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (on DVD), release 2016/17; ASM International: Materials Park, OH, 2016.
- Mykhaylyk V. B.; Kraus H.; Bulyk L.-I.; Lutsyuk I.; Hreb V.; Vasylechko L.; Zhydachevskyy Y.; Wagner A.; Suchocki A. Al2O3 co-doped with Cr3+ and Mn4+, a Dual-Emitter Probe for Multimodal Non-Contact Luminescence Thermometry.. Dalton Trans 2021, 50, 14820–14831. 10.1039/D1DT02836G. [DOI] [PubMed] [Google Scholar]
- Spinolo G.; Gabrielli S.; Palanza V.; Lorenzi R.; Mozzati M. C.; Fasoli M.; Chiodini N.; Vodopivec B.; Paleari A. Mn Sites in Cordierite - Electron Paramagnetic Resonance, Luminescence, and Optical Absorption Analysis. Eur. J. Mineral. 2012, 24, 447–456. 10.1127/0935-1221/2012/0024-2177. [DOI] [Google Scholar]
- Zhang N.; Tsai Y.-T.; Fang M.-H.; Ma C.-G.; Lazarowska A.; Mahlik S.; Grinberg M.; Chiang C.-Y.; Zhou W.; Lin J. G.; Lee J.-F.; Zheng J.; Guo C.; Liu R.-S. Aluminate Red Phosphor in Light-Emitting Diodes: Theoretical Calculations, Charge Varieties, and High-Pressure Luminescence Analysis. ACS Appl. Mater. Interfaces 2017, 9, 23995–24004. 10.1021/acsami.7b06840. [DOI] [PubMed] [Google Scholar]
- Lipinska-Kalita K. E.; Kalita P. E.; Hemmers O. A.; Hartmann T. Equation of State of Gallium Oxide to 70 GPa: Comparison of Quasihydrostatic and Nonhydrostatic Compression. Phys. Rev. B 2008, 77, 094123. 10.1103/PhysRevB.77.094123. [DOI] [Google Scholar]
- Ma Y.-M.; Chen H.-Y.; Yang K.-F.; Li M.; Cui Q.-L.; Liu J.; Zou G.-T. High-Pressure and High-Temperature Behaviour of Gallium Oxide.. Chin. Phys. Lett. 2008, 25, 1603. 10.1088/0256-307X/25/5/022. [DOI] [Google Scholar]
- Remeika J. P.; Marezio M. Growth of Ga2O3 Single Crystals at 44 kbars. Appl. Phys. Lett. 1966, 8, 87. 10.1063/1.1754500. [DOI] [Google Scholar]
- Jovanić B. R. Shift Under Pressure of the Luminescence Transitions of Corundum Doped with Mn4+. J. Lumin. 1997, 75, 171–174. 10.1016/S0022-2313(97)00112-9. [DOI] [Google Scholar]
- Zhydachevskii Ya.; Galanciak D.; Kobyakov S.; Berkowski M.; Kaminska A.; Suchocki A.; Zakharko Ya.; Durygin A. Photoluminescence Studies of Mn4+ Ions in YAlO3 Crystals at Ambient and High Pressure. J. Phys.: Condens. Matter. 2006, 18, 11385–11396. 10.1088/0953-8984/18/49/028. [DOI] [Google Scholar]
- Galanciak D.; Grinberg M.; Gryk W.; Kobyakov S.; Suchocki A.; Boulon G.; Brenier A. Influence of High Pressure on the Luminescence Transitions of Mn4+-doped Gadolinium Gallium Garnet. J. Phys.: Condens. Matter. 2005, 17, 7185–7197. 10.1088/0953-8984/17/46/003. [DOI] [Google Scholar]
- Jara E.; Valiente R.; Bettinelli M.; Rodríguez F. Understanding the Efficiency of Mn4+ Phosphors: Study of the Spinel Mg2Ti1-xMnxO4. J. Phys. Chem. C 2021, 125, 27118–27129. 10.1021/acs.jpcc.1c08006. [DOI] [Google Scholar]
- Forman R. A.; Piermarini G. J.; Barnett J. D.; Block S. Pressure Measurement Made by the Utilization of Ruby Sharp-Line Luminescence. Science 1972, 176, 284–285. 10.1126/science.176.4032.284. [DOI] [PubMed] [Google Scholar]
- Dewaele A.; Torrent M.; Loubeyre P.; Mezouar M. Compression Curves of Transition Metals in the Mbar Range: Experiments and Projector Augmented-Wave Calculations. Phys. Rev. B 2008, 78, 104102. 10.1103/PhysRevB.78.104102. [DOI] [Google Scholar]
- Zimmermann P.; Peredkov S.; Abdala P. M.; DeBeer S.; Tromp M.; Muller C.; van Bokhoven J. A. Modern X-ray spectroscopy: XAS and XES in the laboratory. Coordination Chemistry Review 2020, 423, 213466. 10.1016/j.ccr.2020.213466. [DOI] [Google Scholar]
- Bimberg D.; Sondergeld M.; Grobe E. Thermal Dissociation of Excitons Bounds to Neutral Acceptors in High-Purity GaAs. Phys. Rev. B 1971, 4, 3451. 10.1103/PhysRevB.4.3451. [DOI] [Google Scholar]
- Adachi S. Review - Temperature Dependence of Luminescence Intensity and Decay Time in Cr3+-Activated Oxide and Fluoride Phosphors. ECS J. Solid State Sci. Technol. 2022, 11, 066001. 10.1149/2162-8777/ac7075. [DOI] [Google Scholar]
- Glais E.; Dordevic V.; Papan J.; Viana B.; Dramicanin M. MgTiO3:Mn4+ a Multi-Reading Temperature Nanoprobe. RSC Adv. 2018, 8, 18341–18346. 10.1039/C8RA02482K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski T.; Mahlik S.; Grinberg M.; Liu R.-S. Temperature Effect on the Emission Spectra of Narrow Band Mn4+ Phosphors for Application in LEDs. Phys. Chem. Chem. Phys. 2017, 19, 32505–32513. 10.1039/C7CP06548E. [DOI] [PubMed] [Google Scholar]
- Senden T.; van Dijk-Moes R. J. A.; Meijerink A. Quenching of the red Mn4+ luminescence in Mn4+-doped fluoride LED phosphors. Light Sci. Appl. 2018, 7, 8. 10.1038/s41377-018-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle K. A.; Kioupakis E. Vibrational and Electron-Phonon Coupling Properties of β-Ga2O3 from First-Principles Calculations: Impact on the Mobility and Breakdown Field. AIP Advances 2019, 9, 015313. 10.1063/1.5055238. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Villars P.; Cenzual K.. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (on DVD), release 2016/17; ASM International: Materials Park, OH, 2016.















