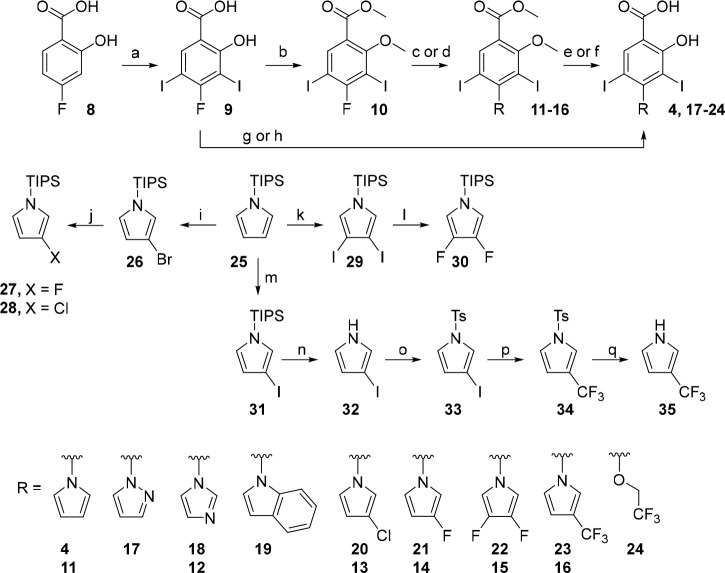
Scheme 1. SNAr Chemistry toward Subsite 3 Iodophenol Analogues.
Conditions: (a) NIS, AcOH, 25 °C, 6 h, 86%; (b) K2CO3, Me2SO4, NMP, 80 °C, 1 h, 68%; (c) heterocycle, Cs2CO3, DMSO, 70 °C, 1 h, 45–68%; (d) KF, 1-TIPS-pyrrole derivative, Cs2CO3, DMSO, 70 °C, 1 h, 73–80%; (e) BBr3, CH2Cl2, 0 to 25 °C, 24 h, 27–62%; (f) TMSI, CH2Cl2, 50 °C, sealed tube, 24 h, 31%; (g) Cs2CO3, heterocycle, DMSO, 150 °C, 2 h, 55–58%; (h) CF3CH2OH, NaH, DMF, 0 to 150 °C, 20 h, 22%; (i) NBS, THF, −78 to 25 °C, 3 h, 89%; (j) nBuLi, NFSI or NCS, THF, −78 to 25 °C, 1.5 h, 48–50%; (k) I2, H5IO6, Et2O, 25 °C, 1 h, 86%; (l) nBuLi, NFSI, THF, −78 to 25 °C, then nBuLi, NFSI, THF, −78 to 25 °C, 1 h, 32%; (m) NIS, acetone, −78 to 25 °C, 5 h; (n) TBAF, THF, 25 °C, 1 h; (o) NaH, TsCl, THF, 0 to 25 °C, 1 h, 84% over 3 steps; (p) FSO2CF2COOCH3, CuI, HMPA, DMF, 80 °C, 16 h, 62%; (q) Mg, MeOH, 25 °C, 0.5 h, 73%.