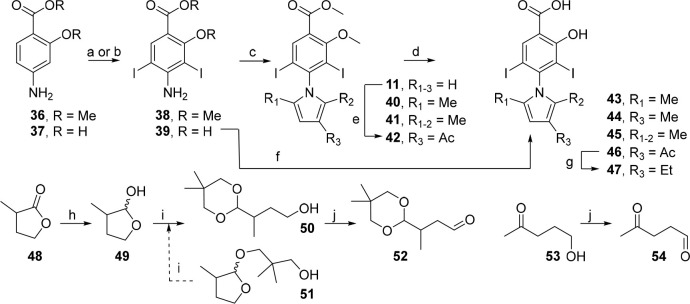
Scheme 2. Carbonyl Condensation Chemistry toward Subsite 3 Iodophenol Analogues.
R1–3 = H unless defined. Conditions: (a) NIS, AcOH, 25 °C, 1 h, 98%; (b) NIS, MeCN, 25 °C, 0.5 h, quant.; (c) dicarbonyl, conc. aq. HCl (cat.), EtOH, reflux, 18 h, 22–35%; (d) BBr3, CH2Cl2, 0 to 25 °C, 24 h, 27–59%; (e) Ac2O, BF3·OEt2, CH2Cl2, 0 to 25 °C, 2 h, 57%; (f) 52, AcOH, 100 °C, 6 h, 26%; (g) Et3SiH, TFA, 50 °C, 1 h, 48%; (h) DIBAL-H, Et2O, −78 °C, 30 min, 81%; (i) neopentyl glycol, TsOH·H2O, PhMe, 100 °C, 2 h, 46–50% 50, 19–28% 51; (j) SO3·py, Et3N, DMSO, CH2Cl2, 0 to 25 °C, 15 h, 69%–quant.