FIGURE 4.
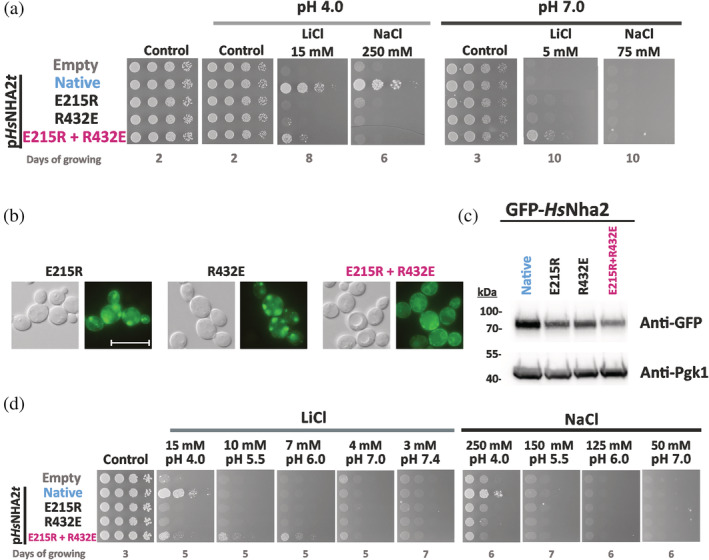
Characterization of HsNHA2 with mutations of residues forming a putative salt bridge. (a) Salt tolerance of S. cerevisiae BW31 cells containing empty vector or expressing native HsNHA2 or its mutated versions (E215R, R432E or E215R + R432E) from pHsNHA2t plasmid. Cells were grown on YNB‐Pro (pH approx. 4.8) or non‐buffered YNB‐Pro plates with the pH adjusted to 4.0 or 7.0 and supplemented with LiCl or NaCl as indicated. Plates were incubated at 30°C and photographed on the indicated day. Localization (b) and immunodetection (c) of N‐terminal GFP‐tagged HsNHA2 mutated versions E215R, R432E or E215R + R432E. Cells were grown in YNB‐Pro (4% glucose) to the exponential phase and observed under a fluorescence microscope (b, right). A Nomarski prism was used for whole‐cell imaging (b, left). The scale bar corresponds to 10 μm. In (c), protein extracts from the same cells as in (b) (with cells expressing native GFP‐HsNHA2 as a control) were prepared as described in Section 5, subjected to SDS‐PAGE (10% gel) and transferred to a nitrocellulose membrane. GFP‐HsNHA2 was detected with an anti‐GFP antibody. The membrane was reprobed and incubated with an anti‐Pgk1 antibody to verify the amount of loaded proteins. (d) Activity of mutated HsNHA2 antiporters at various extracellular pH levels determined by growth of cells on YNB‐Pro plates buffered to various pH levels (4.0–7.4) and supplemented with LiCl or NaCl as indicated. Images were taken on the indicated day of incubation at 30°C
