Stoichiometric C–H bond pentafluoroethylation by 1a.
| Entry | Substrate | Conditions | Yield [%] |
|---|---|---|---|
| 1 |
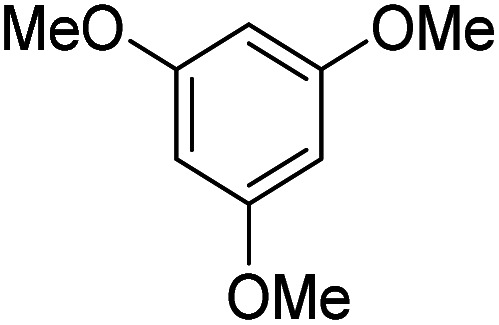
|
— | 0 |
| 2 |
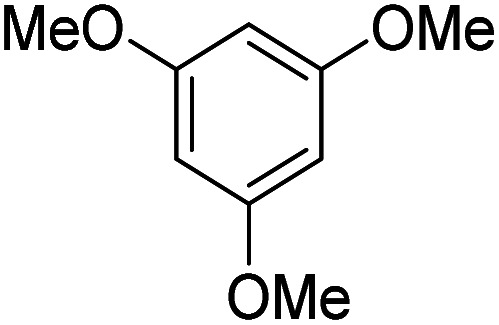
|
K2S2O8 (1 equiv.) | 75 |
| 3 |
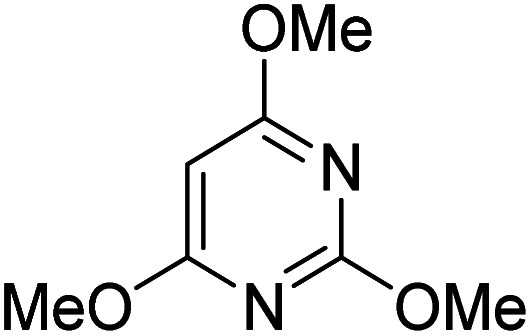
|
K2S2O8 (1 equiv.) | 80 |
| 4 |
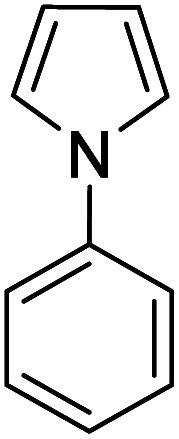
|
K2S2O8 (1 equiv.) | 38 |
| 5 |
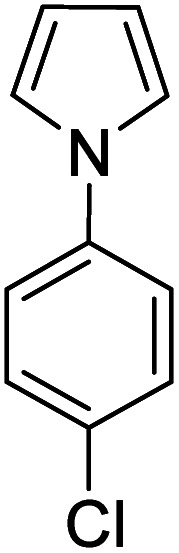
|
K2S2O8 (1 equiv.) | 50 |
| 6 |
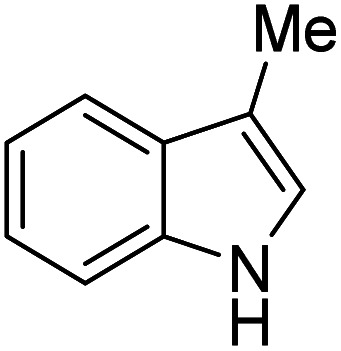
|
K2S2O8 (1 equiv.) | 37 |
Reactions were performed for 24 hours using 1 equiv. of the substrate, and 1 equiv. of 1 in DMSO at RT under N2 unless indicated otherwise. The yields were determined by 19F NMR based on integration against α,α,α-trifluorotoluene as an internal standard.
