Table 3.
Epidural electrical spinal stimulation studies demonstrate stimulation within lower-thoracolumbar and sacral spinal regions can ameliorate multiple spinal sympathetic dysfunction(s)
| Study | Protocol | Electrode Placement and Configuration; Stimulation Parameters and Stimulator | Autonomic Outcome(s) |
|---|---|---|---|
Squair et al. (30)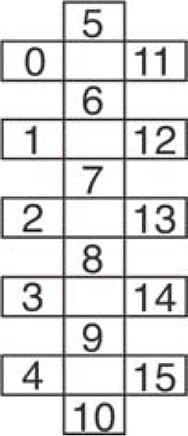
|
1) Simulation parameters that elicited a rise in resting systolic BP compared. Selected parameters eliciting ↑ of ∼40 mmHg for use in orthostatic challenge (OC) in step 2. Then: 2) OC with and without ESCS |
T10–L1 D, S Electrode: 3-Col (5-6-5 lead) 16-contact array Cathode (C): 0–1, 11–12 Anode (A): 5–6 Freq: 120 Hz Amp: 0–7.5 mV Width: 450 µs Intensity determined by degree of reduction in BP during orthostatic hypotension (OH) Stimulator: Medtronic RestoreAdvanced SureScan neurostimulator |
ESCS normalized OH-induced decreases in BP and could increase resting systolic BP by ∼40 mmHg 1) Testing configurations for ↑ systolic BP at rest: i) ESCS ↑ resting systolic BP by ∼40 mmHg: C: 0–1, 11–12 A: 5–6 ii) ESCS ↑ resting systolic BP by ∼13 mmHg: C: 2, 13 A: 8 iii) ESCS ↑ resting systolic BP by ∼5 mmHg: C: 3–4, 14–15 A: 9–10 iv) ESCS ↑ resting systolic BP by ∼0 mmHg: C: 5–6 A: 0–1, 11–12 Configuration i) selected as most effective for f↑ BP, and used during OC. 2. ESCS could repeatedly interrupt episodes of OH during OC. Other: Reported serum noradrenaline increased from ∼0.2 to 0.6 nmol/L after ESCS |
Darrow et al. (31)
|
1) Implantation of electrode used intraoperative monitoring of leg muscle EMG and was secured in place where maximal and symmetric EMG observed with lowest stimulation current. 2) BP and HR were monitored during orthostatic challenge (OC: 70 degrees tilt-table test). SCS applied once orthostatic hypotension (OH) observed with and without SCS. |
L1–S2 D, S Electrode: 3-Col (5-6-5 lead) 16-contact array Freq: 50 Hz Amp: 5 mA Width: 350 µs Participant (P)1 (no OI): C: 0, 6, 11 A: 4, 10, 15 P2 (OI): C: 0, 5, 11 A: 4, 9, 15 Stimulator: Primary cell internal pulse generator (Tripole and Proclaim Elite). |
SCS normalized cardiovascular responses during OC in participant with OI and did not alter responses in person without OI P1 (no OI):
|
Nightingale et al. (19)
|
Six progressive arm crank ergometry tests to exhaustion (V̇o2peak), separated by 12 days. Each of 3 conditions [1) no ESCS, 2) abdominal (AB) ESCS or 3) cardiovascular (CV) ESCS repeated twice]. Order of testing randomly assigned with assessors blinded as to trial allocation. |
T11–L1 D, V Electrode: 3-Col (5-6-5 lead) 16-contact array AB parameters: Freq: 40 Hz Amp: 3.5–6.0 V Width: 420 µs C: 1, 6, 12 A: 0, 5, 11 CV parameters: Freq: 35 Hz Amp: 3.5–6.0 V Width: 300 µs C: 1, 4, 6–10, 12, 15 A: 0, 2, 3, 5, 11, 13, 14 * same participant as in 34 |
Exercise related autonomic outcomes
Both AB and CV ESCS: ↑ V̇o2peak (absolute and relative; 15%–26%) ↑ Peak ventilation (from 33 to ∼50 L/min with SCS) ↑ Peak oxygen pulse (8%–13 % for low intensity SCS, 21% for high intensity SCS) ↓ RPE at sub-peak power output of 60 W (from 18/20 to 14–15/20) In addition, CV ESCS: ↑ MAP by 14 mm Hg at rest (cardio program) regardless of intensity |
Aslan et al. (32)
|
1) Before electrode implantation, orthostatic intolerance (OI) was determined as follows. While supine, blood was drawn for baseline serum catecholamine concentrations, then repeated at 3′ and 10′ of sitting orthostatic challenge (OC). BP and HR were monitored continuously throughout testing. Participants were separated into two groups: 1) OI, if they demonstrated resting hypotension, OI to sitting challenge and low levels of circulating catecholamines or 2) no OI, if the above were not observed. 2) Monitored resting supine BP and leg EMG responses to increasing ESCS amplitude at each of rostral and caudal ESCS configurations. 3) Monitored BP and HR during ESCS-induced standing before and after transition from sit to stance. Stimulation parameters and electrode configuration individually determined but selected specifically to elicit motor activity with cathode in caudal ESCS configuration. |
L1–S1 D, S (T11–L1 V) Electrode: 3-Col (5-6-5 lead) 16-contact array. C, A, and Freq Individualized*. Grp 1 *B23: 30 Hz, C = 7, 10, 13, A = 2, 4, 15 *B13: 15 Hz, C = 4, 10, 15, A = 9 *B07: 15 Hz, C = 4, 10, 15, A = 3, 9, 14 Grp 2 *A60: 25 Hz, C = 4, 10, 14, A = 3, 12 *A59: 25 Hz, C = 4, 10, A= 6, 12 *A53: 35 Hz, C = 4, 10, 15, A = 3, 8, 14 *A45: 25 Hz, C = 4, 10, 14, A = 3 Stimulator: Medtronic 5-6-5 Specify with RestoreADVANCED pulse generator |
ESCS normalized cardiovascular responses during OC in group with OI and did not alter responses in group without OI
Group 1 (OI: all AIS B, C5–T2 SCI)
|
| DiMarco et al. (33) |
1) Electrode implantation to restore cough function. 2) Participant instructed to apply stimulation every 30 s for 5–10 min, 2 or 3 times/day, in the home to help with expiratory airflow. 3) Participant was able to cough when using SCS. Peak expiratory airflow rate and maximum pressure increased and plateaued over 6–8 wk. Was followed for > 1.5 yr. |
T9–T11 D, S, just lateral to midline on L and R Freq: 50 Hz Amp: 40 V Width: 0.2 ms Waveform: biphasic Electrode: 4 lead, bilateral Implanted receiver (Finetech Medical Ltd) activated by an external transmitter, controlled by a portable hand-held stimulator. |
Anecdotal Data
BP ↑ to 175 mmHg in first ESCS session HR ↓ to 55 beats/min in first ESCS session Cardiovascular responses gradually abated and then disappeared with repeated use over a 9-wk period. |
Harkema et al. (15)
|
1) Stimulation parameters and electrode configuration individually determined over 2–3 initial 2-h sessions while at seated rest with outcome goal of 105–120 mmHg SBP, while minimizing EMG in multiple leg muscles. 2) Repeat testing of “effective” stimulation leads/parameters on BP, HR at rest over 5 subsequent 2-h sessions. |
L1–S1 D, S (T11–L1 V) Freq: 30–65 Hz Amp: 3–7 V; selected based on sys BP of 105—120 mmHg while < motor Thr Width: 450 µs Electrode: 3-Col (5-6-5 lead) 16-contact array. C, A and Freq Individualized*. Stimulator: Medtronic 5-6-5 Specify with RestoreADVANCED pulse generator |
ESCS normalized resting BP in persons with cSCI and OI
|
West et al. (34)
|
1) Using epidural stimulator implanted to improve lower limb stepping function, stimulation parameters, and electrode configuration to increase BP while at seated rest was determined with initial testing over a 2-wk period. Outcome goal of 105–120 mmHg SBP, while minimizing EMG in multiple leg muscles. 2) 3 testing days consisted of supine and head up tilt manipulation with and without SCS while monitoring beat by beat BP, cardiac function with transthoracic echocardiography, cerebral blood flow with transcranial Doppler, and lower limb EMG. |
T11–L1 D, V Electrode: 3-Col (5-6-5 lead) 16-contact array C: 1, 4, 6-10, 12, 15 A: 0, 2-3, 5, 11, 13-14 Freq: 35 Hz Amp: 3.5 V Width: 300 ms Stimulator: Medtronic 5-6-5 Specify with RestoreADVANCED pulse generator |
ESCS normalized cardiovascular responses during orthostatic challenge
ESCS:
|
| Edgerton and Harkema (35) | Review and commentary of >18-mo observations using epidural stimulation in motor complete T1 participant with SCI. | L1–S1 D, S (T10–T12 V) Electrode: 16-leads Freq: NR Amp: NR Width: NR Stimulator: NR |
Exercise related autonomic outcomes
|
| Ganley et al. (36) | Protocol as in Carhart (37) and Herman (16) and includes same participant (P1) + 1 additional participant 2 (P2) had T8 SCI and received FES during PBWT before receiving epidural implant. | T10–T12 D, V, but for P2, leads were placed slightly more lateral due to scar tissue. Freq: 20–60 Hz Amp: Midway between sensory and motor threshold (Thr) for P1 and at motor Thr for P2. Width: 800 µs Stimulator: as in Carhart and Herman |
Exercise related autonomic outcomes
Overground walking with ESCS at each time point tested showed immediate changes for P1 as described in Carhart and Herman. P2 showed similar but less robust effects on speed and endurance as P1. In addition, P2 showed:
|
| Carhart et al. (37) |
1) Treadmill: partial weight bearing therapy (PWBT), < 2 h/day, 5 times/wk until plateau (from 0.45 m/s, 40% static BW to 0.65 m/s < 20% BW) 2) Treadmill-PWBT with/without ESCS 3) Overground training with/without ESCS |
Lumbar enlargement, D, S (T10–T12 V) Electrode: 4 cylindrical contacts (6 mm × 1.2 mm), 2 leads arranged in parallel, 1 mm lateral to midline, quadripolar stimulation configuration Freq: 40–60 Hz Amp: Midpoint between sensory and motor Thr Width: 800 µs Waveform: Continuous, charge-balanced monophasic rectangular pulse followed by low amplitude-long duration opposite polarity rectangular pulse.* Stimulator: Medtronic fitted with dual Pisces-Quadplus Model 3888 electrode leads, X-TREL 3470 receiver, X-TREL transmitter (M 3425) and external antenna (M 3440). Transmitter powers implanted receiver via transcutaneous radio frequency telemetry. Pulse generator used in SingleStim mode. |
Exercise related autonomic outcomes
Overground walking with ESCS at each time point tested showed immediate:
|
| Herman et al. (16) |
1) Established plateau in gait performance (90% BW, 2.0 mph) using progressive training with partial weight bearing therapy (PWBT) on a treadmill. 2) Implant epidural stimulator, after healing, re-implement PBWT with ESCS. 3) Compare mean speed, stepping symmetry, RPE, and whole body metabolic activity with and without ESCS during stepping. Motor: with ESCS smoother stepping pattern at higher treadmill speeds and self-supported body weight, less spasticity. |
T11–T12 V 4 contacts, placed 1–2 mm off dorsal midline with span of contacts covering 15 mm to span the entire upper lumbar enlargement. Freq: 20–60 Hz (observed similar responses over this range of frequencies) Amp: > Sens Th, < Mot Thr Width: 800 µs lower durations less effective Stimulator: Medtronic fitted with a pair of Pisces-Quadplus electrodes (with X-TREL stimulation system, Medtronics) inserted into the dorsal epidural space. |
Exercise related autonomic outcomes
Overground walking with ESCS:
|
Studies focus on autonomic, motor, or autonomic and motor outcomes. The configuration of lead(s) selected as cathode (C) and anode (A) in each study and/or for each participant are included in columns 3 or 4, if provided. Ab, abdominal; BP, blood pressure; BW, body weight; cMAP, cerebral mean arterial pressure; Co, coccygeal; CO, cardiac output; D, dorsal; ESCS, epidural spinal cord stimulation; FA, fatty acid; FM, fat mass; HI, high intensity; MAP, mean arterial pressure; mCBFv, mean cerebral blood flow velocity; NR, not reported; OC, orthostatic challenge; OH, orthostatic hypotension; OI, orthostatic intolerance; PWBT, partial weight-bearing training; RER, respiratory exchange ratio; RPE, rating of perceived exertion; S, spinal; SCS, spinal cord stimulation; SV, stroke volume; thr, threshold.
