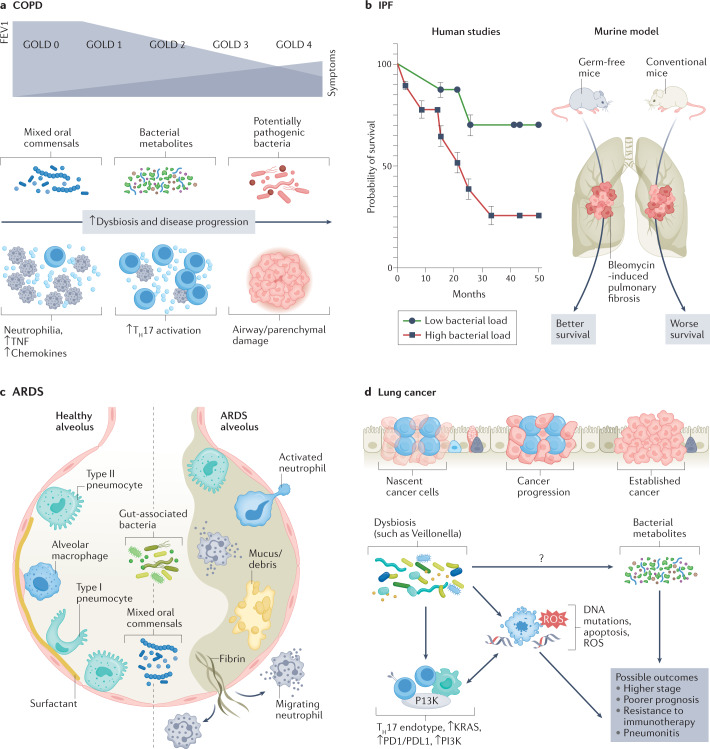
Fig. 3. Disease-specific dysbiotic signals.
Different forms of dysbiosis coexist with different immune endotypes and pathological phenotypes in various disease states. a, In chronic obstructive pulmonary disease (COPD), progression to advanced stages, characterized by decrease in forced expiratory volume in one second (FEV1) and higher Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, is hallmarked by the presence of potentially pathogenic microbes, neutrophilia, and increased inflammatory cytokines and chemokines. Newer investigations are identifying early dysbiotic events involving some oral commensals and bacterial metabolites that might precede the development of advanced-stage COPD. b, Increased bacterial burden has been shown to be associated with a poorer prognosis in patients with idiopathic pulmonary fibrosis (IPF). In animal studies, germ-free mice had a survival benefit after the induction of bleomycin-induced pulmonary fibrosis as compared to conventional mice with normal bacterial burden. c, In acute respiratory distress syndrome (ARDS), there is a relationship between gut-associated bacteria and mixed oral commensals with alveolar filling, increased mucus or cellular debris, and immune activation. d, Mixed oral commensals can play a role in the progression of lung cancer by perpetuating a T helper 17 (TH17) endotype, increased PI3K signalling and immune-checkpoint inhibition. These events, in conjunction with the more direct carcinogenesis precursors (such as DNA mutations and cell apoptosis, which further contribute to a pro-inflammatory tumour microenvironment), can have serious effects on the prognosis of lung cancer. It is likely that metabolites regulated by microbial metabolism play a significant role in cancer pathogenesis, which deserves further investigation. ROS, reactive oxygen species.