Highlights
-
•
Baseline resting brain perfusion was elevated in several language-related areas in first episode psychosis, indicating over-activity in language and semantic related areas.
-
•
Baseline resting perfusion was reduced in several executive control regions in first episode psychosis, indicating underactivity of higher-order cognitive control areas.
-
•
Basal perfusion differences in first episode psychosis were not markedly different between hallucinators and non-hallucinators, suggesting these patterns were associated broadly with emerging psychosis.
Keywords: First episode psychosis, Basal perfusion, Arterial spin labeling, Auditory hallucinations
Abstract
Background and hypothesis
Cortical (e.g., Broca’s area and Wernicke’s area) and subcortical (e.g., putamen) language-related areas and executive control areas (e.g., inferior frontal gyrus (IFG), dorsolateral prefrontal cortex (DLPFC)) show functional and structural dysconnectivity in long-term psychosis. We examined whether resting-state basal perfusion levels revealed selective pathophysiology (likely hypo- and hyper-activation) of language-related and executive areas in first-episode psychosis (FEP).
Study design
Basal resting-state perfusion was measured using pseudo-continuous Arterial Spin Labeling (pcASL). Relative cerebral blood flow (rCBF) was compared between 32 FEP and 34 matched healthy comparison (HC) individuals. Structural and functional MRI scans were acquired using a 3T Prisma scanner during the same session.
Study results
Whole-brain comparison of resting rCBF identified 8 clusters with significant between-group differences. Reduced rCBF was found in executive control areas in left and right IFG, right DLPFC, and right parietal cortex. Increased rCBF was found in left and right temporal cortex (including Wernicke’s area), and left and right putamen. A positive correlation was observed between auditory hallucination severity and rCBF in the left putamen.
Conclusions
To the degree that perfusion implies activation, language and auditory processing areas in bilateral temporal lobe and putamen showed pathological hyper-activity, and cognitive control areas (IFG, DLPFC, right parietal) showed pathological hypo-activity in FEP at rest. Pathological basal activity was present across the range of symptom severity, suggesting it may be a common underlying pathology for psychosis that may be targeted with non-invasive brain stimulation to normalize resting activity levels.
1. Introduction
Cross-diagnostic analyses of high- and low-base rate psychiatric disorders have identified three largely orthogonal dimensions: Internalizing, Externalizing, and Psychosis (Wolf et al., 1988, Kotov et al., 2011, Wright et al., 2013, Caspi et al., 2014). Psychosis splits into positive and negative symptom components, and those components parse into more refined constructs. Positive symptoms can be further refined into reality distortion and thought disorder. Reality distortion comprises auditory hallucinations (AH) and delusions. We examined resting-state perfusion, a putative measure of hyper- and hypo-activity of brain regions, and its association with AH severity using a data-driven whole brain approach. AH are prevalent, affecting roughly 70% of individuals with schizophrenia (Mueser et al., 1990, McCarthy-Jones et al., 2017), even at first-episode (Salisbury et al., 2020). Estimates of AH in bipolar disorder and depression are variable, and range from 10 to 50% (see review (Toh et al., 2015). Our data in over 100 first-episode affective psychosis (mainly mania) individuals found about 50% experienced AH (Salisbury, unpublished data). Auditory perceptual abnormalities are also highly expressed in individuals at clinical high risk for psychosis (CHR, >70%) (Addington et al., 2015). Thus, AH and auditory misperceptions are common symptoms expressed across diagnostic categories that are particularly debilitating in psychotic disorders. If AH were, at least in part, related to functional hypo- or hyper-activity in cortical areas rather than specific loci of structural gray or white matter loss, identifying system-level pathophysiology underlying AH would provide novel targets for interventional psychiatry via modulation of basal neural activity with non-invasive brain stimulation which affects resting membrane potentials.
Several studies suggest that the left temporal cortex has a high basal rate of activity in participants with AH (Dierks et al., 1999, Wolf et al., 2012, Kindler et al., 2013). Increased PET blood flow in left hemisphere secondary auditory cortices in hallucinating subjects normalized with resolution of AH and returned with relapse (Suzuki et al., 1993). Further, brain stimulation with repetitive Transcranial Magnetic Stimulation (rTMS) produces long-lasting reductions of AH by reducing basal activity levels in left temporal-parietal language areas (Hoffman et al., 2000, Hoffman et al., 2003, Hoffman et al., 2007). Additionally, basal activity levels demonstrated by arterial spin labeling (ASL) are reduced in a distributed semantic circuit including Wernicke’s area, Broca’s area, and primary auditory cortex after rTMS (Kindler et al., 2013). Both Wernicke’s and Broca’s areas are over-activated during hallucinations (Lennox et al., 2000), and increased fMRI functional connectivity between these areas has been demonstrated in AH (Hoffman et al., 2011). Additionally, several studies have shown that primary auditory cortex (PAC) is activated during AH (Dierks et al., 1999, Lennox et al., 2000, Strik et al., 2008). Thus, functionally, AH appear to be associated with over-activation within a left hemisphere dominant language/semantic circuit. Based on functional connectivity in fMRI during a resting task, Hoffman et al (Hoffman et al., 2011) argued that imbalance (whether hyper- or hypo-connectivity) between three legs of a Broca’s-Wernicke’s-putamen circuit lead to AH. The authors argued that the putamen plays a critical role in activating language representations in semantic memory. Functional connectivity between Wernicke’s and Broca’s areas was increased in AH compared to non-AH patients, but not when compared to healthy controls (HC). Wernicke’s area also showed increased coupling to putamen in both patient groups. Finally, Broca’s area showed increased coupling with putamen in AH but not in non-AH. In sum, Hoffman et al (Hoffman et al., 2011) suggested that overall functional connectivity in the loop was elevated in AH individuals, but not in non-AH individuals, compared to HC.
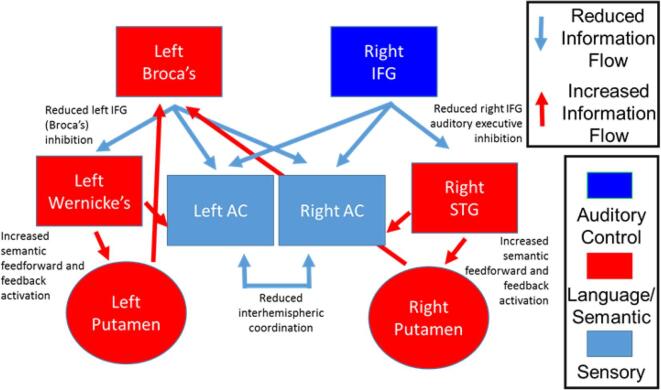
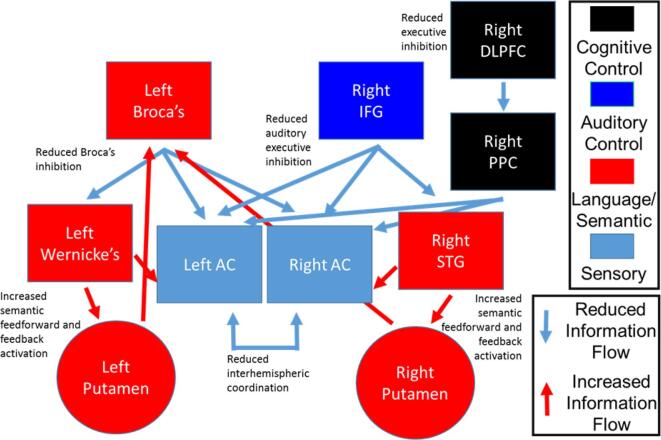
Our hybrid model of the language-related circuit and AH combines elements of semantic memory intrusion, bottom-up sensory and top-down cognitive control imbalance (which includes source monitoring deficits), and interhemispheric dysconnectivity. The model includes contributions from both hemispheres, and comprises Broca’s area and the right inferior frontal gyrus (IFG) homologue, Wernicke’s area/temporal parietal junction (TPJ) and the right hemisphere homologue, bilateral putamen, the bilateral auditory cortices, and the white matter tracts connecting them (Fig. 1). Our hypothesis is that reduced IFG-mediated (Broca’s) control of auditory activity causes an imbalance of frontal-temporal functional connectivity within the language circuit that impinges on auditory perception and semantic memory by causing over-activation of Wernicke’s area/TPJ. This overactivity, in turn, leads to spurious activation of upstream auditory cortices (perception) and downstream semantic stores (memory), leading to auditory perceptual aberrations and AH. Additionally, deficient interhemispheric communication between auditory/language areas leads to disordered coordination of auditory and semantic processing, further compounding the spurious sensory and semantic activations leading to AH. The major fiber bundle connecting frontal and parietal language areas is the arcuate fasciculus (AF). Based on the functional connectivity data of Hoffman et al (Hoffman et al., 2000, Hoffman et al., 2003, Hoffman et al., 2007, Hoffman et al., 2011) that identified a role of the putamen of the basal ganglia in AH, subcortical pathways between left and right IFG and putamen, and bilateral Wernicke’s area/TPJ and putamen are also present. Interhemispheric communication between auditory cortices is an important aspect of perceptual aberrations and serves as the major inter-hemispheric connection in our model.
Fig. 1.
Systems-level model of auditory hallucinations incorporation bottom-up sensory deficits, top-down auditory executive control deficits, and semantic memory intrusion deficits. (After circuit model in (Salisbury et al., 2021).
In a previous report examining white matter structural connectivity in this “Hoffman Hallucination Circuit”, we detected white matter diffusion abnormalities in white matter fibers connecting Broca’s area and Wernicke’s area that correlated with AH severity in FEP (Salisbury et al., 2021). These white matter microstructural deficits are consistent with miscommunication between top-down and bottom-up nodes of the language system and consequent spurious semantic memory activation in FEP. We also observed marked white matter deficits in callosal connections between auditory cortices that correlated with AH severity, consistent with reduced information transfer and failure of coordination and information integration between hemispheres. To expand on these structural findings, here we examined basal perfusion in an independent sample of FEP using pseudo-continuous arterial spin labeling (pcASL). To the extent that basal perfusion reflects activity levels in brain areas, we aimed to determine whether early psychosis is characterized by hyper- and hypo-activity of critical language circuit nodes, and whether such pathophysiology correlates with AH. We hypothesized that emerging psychosis is characterized by hyper-perfusion of posterior temporal auditory/language areas and putamen coupled with hypo-perfusion of frontal and parietal cognitive control areas and that greater pathophysiology would lead to increased AH severity. If correlations between pathophysiology and AH exist, then the abnormal activity indicates dysconnectivity specifically related to the auditory/semantic system. If the pathophysiology exists independent of AH severity, then the abnormal activity likely reflects dysconnectivity more fundamental to the emergence of all facets of psychosis. We used a whole brain data-driven approach to identify any area that showed basal perfusion abnormalities not included in our circuit model. Further, we hypothesized that the pathophysiology would be reflected in impaired cognition and social functioning, domains highly impacted by psychosis.
2. Methods
2.1. Participants
Thirty-two FEP and 34 HC individuals participated, group-matched for mean age, sex distribution, verbal IQ assessed by the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999), and parental socioeconomic status (pSES (Hollingshead, 1975), see Table 1 for demographic measures). Participants were excluded for a) history of concussion or head injury with sequelae, b) history of alcohol or drug dependence, or detox in the last five years, c) presence of neurological disease or disorder, d) <9 years education, or e) estimated IQ <75. Participants provided voluntary informed consent and were compensated for participation. Procedures were in accord with the Declaration of Helsinki and were approved by the University of Pittsburgh Institutional Review Board.
Table 1.
Subject Demographics and Clinical information.
| FEP | HC | t/X2 | p | |
|---|---|---|---|---|
| N | 32 | 34 | ||
| M/F | 19/13 | 22/12 | 0.199 | 0.655 |
| Age | 23.6 (4.7) | 24.9 (5.3) | 1.042 | 0.301 |
| WASI Verbal t-score | 50.0 (9.9) | 53.0 (6.6) | 1.447 | 0.153 |
| MATRICS Composite t-score | 34.0 (13.7) | 50.8 (7.6) | 6.222 | <0.001 |
| Education (years) | 12.9 (3.1) | 16.0 (2.8) | 4.249 | <0.001 |
| Socio-Economic Status | 32.1 (14.7) | 42.8 (12.5) | 3.167 | 0.002 |
| Parental Socio-Economic Status | 44.9 (13.0) | 49.4 (9.9) | 1.549 | 0.126 |
| Role current | 5.3 (1.9) | 9.1 (0.2) | 11.619 | <0.001 |
| Role lowest past year | 5.2 (2.0) | 9.0 (0.0) | 11.237 | <0.001 |
| Role highest past year | 7.5 (1.5) | 9.1 (0.2) | 5.862 | <0.001 |
| Interpersonal current | 5.3 (2.0) | 9.1 (0.2) | 10.662 | <0.001 |
| Interpersonal lowest past year | 5.0 (1.9) | 9.0 (0.1) | 12.161 | <0.001 |
| Interpersonal highest past year | 7.5 (1.3) | 9.1 (0.2) | 7.243 | <0.001 |
| PANSS total | 74.7 (18.6) | |||
| PANSS Positive | 20.4 (6.5) | |||
| PANSS Negative | 16.8 (5.6) | |||
| PANSS General | 37.5 (9.2) | |||
| SAPS Global | 5.8 (1.9) | |||
| SAPS AH component | 3.7 (3.6) | |||
| SANS Global | 8.8 (4.6) | |||
| Medicated/Unmedicated | 21/11 | |||
| Medication only (mean CPZ equivalents) | 288.5 (177.2) | |||
| First clinical contact to scan (mean months) | 3.8 (9.3) | |||
| First clinical contact to scan (median months) | 1.1 | |||
| DUP (mean years) | 1.2 (1.9) | |||
| DUP (median years) | 0.5 | |||
Diagnosis utilized the Structured Clinical Interview for DSM-IV (SCID-IV) and consensus conference review. Provisional research diagnoses were initially made at baseline, and then confirmed 5–7 months later based on follow-up diagnostic interviews and review of all available longitudinal data. Symptoms were rated using the Positive and Negative Symptom Scale (PANSS), the Scale for the Assessment of Positive Symptoms (SAPS), and the Scale for the Assessment of Negative Symptoms (SANS). PANSS total, positive, negative, and though disorder factors were calculated. To investigate auditory hallucinations more deeply, an Auditory Hallucinations component score based on a hierarchical principal component analysis of SAPS scores was calculated (Longenecker et al., 2022), comprising Q1. Intensity of Auditory Hallucinations, Q2. Intensity of Voices Commenting, and Q3. Intensity of Voices Conversing. All interviews and tests were conducted by an expert (Masters’- or PhD-level) clinical assessor. Of the 32 FEP participants, 19 received diagnoses of Schizophrenia (10 paranoid, 1 residual, 8 undifferentiated), 1 of Schizoaffective Disorder, 2 of Schizophreniform (definite), 2 of Psychotic Disorder NOS, 5 of Bipolar Disorder with psychotic features, and 3 of Major Depressive Disorder with psychotic features. All FEP participated shortly after their first clinical contact for psychosis, and all (apart from one) had <3 months of lifetime antipsychotic medication exposure (schizophreniform diagnosis; <1yr total exposure; analysis was run with and without this participant, and no differences were found in the results). See Table 1 for clinical measures.
3. Procedures
Participants underwent clinical interviews as described above and social functioning was rated with the Global Functioning Scales (Cornblatt et al., 2007). Participants also underwent neuropsychological assessments. Batteries included the Wechsler Abbreviated Scale of Intelligence, with Verbal IQ used as an index of premorbid intellectual functioning (∼trait intellect), and the MATRICS battery, comprising tests sensitive to the negative effects of psychosis on current cognition (∼state intellect). Participants underwent MRI scanning on a Prisma 3T scanner. In addition to the HCP protocol (T1, T2, rsfMRI) and DSI, participants underwent pcASL scanning with a 2D simultaneous multislice echo planar imaging (SMS-EPI) sequence with TR = 4.9 s, TE = 16 ms, voxel size = 3.3×3.3×4 mm, labeling offset = 90 mm, labeling duration = 1800 ms, post labeling delay = 1800 ms, 30 averages, 25 slices with 1 mm gap, and 5 multi-band acceleration. Briefly, pcASL compares a “control” perfusion image with a “labeled” image after magnetizing carotid arterial blood. Prior to each scan, participants gave verbal confirmation they were awake and ready for the scan, and were reminded to stay awake. After the session they were asked if they ever fell asleep during scanning.
Motion correction was performed by realignment of the control and label image series to the M0 image. Then, zig-zagged patterns (e.g. label = −1 and control = 1) were regressed out from the motion regressor time-series (Wang et al., 2008). A structural correlation-based outlier rejection method was applied to remove artifacts arising from outlier control-label pairs in ASL data using structural correlation based outlier rejection (SCORE) alogorithm (Dolui et al., 2017). Volumes outside 2.5 SD of the mean were discarded. The perfusion-weighted maps were calculated voxel by voxel from the average of the signal differences between labeling and control images. An adaptive optimised non-local mean filter was applied to the perfusion-weighted map using a Gaussian model for denoising (Manjón et al., 2010). The CBF was quantified by (6000⋅λ⋅ΔS⋅exp(-PLD/T1,blood))/(2⋅α⋅T1,blood⋅SPD⋅(1-exp(-τ/T1,blood)), where λ is the brain/blood partition coefficient (=0.9 mL/g), ΔS is the perfusion-weighted signal (control – label), T1,blood is the longitudinal relaxation time of blood in seconds (=1.65 s), α is the labeling efficiency (=0.85) (Alsop et al., 2015a), SPD is the signal intensity of a proton density-weighted image, and τ is the label duration (Alsop et al., 2015b). For whole brain voxel-wise analysis, the pcASL images were registered to the T1-weighted anatomical image and normalized to MNI space. The CBF maps were normalized by mean CBF value of gray matter and a 3D Gaussian filter was applied.
3.1. Statistical analysis
Voxel-wise rCBF was first compared between groups using threshold-free cluster enhancement (TFCE) as implemented by FSL. TFCE accounts for multiple comparisons. For group comparison, 5000 permutations were generated to identify statistically significant voxels while controlling family-wise error rate (p < 0.05) (Winkler et al., 2014). Age and sex were used as covariates. Contiguous regional clusters identified as significantly different between HC and FEP. Clusters that spanned more than 32 voxels were then used as masks to extract average regional rCBF values from individuals. These values were used for group and sub-group comparisons. Finally, to investigate relationships between ROI rCBF, symptoms, and functioning, Spearman’s correlation was employed. To capitalize on the rich dataset, and realizing the hypothesis-driven and exploratory nature of the symptom, cognitive, and functioning correlations, results were considered significant with p < 0.05, and will need replication.
4. Results
Groups did not differ in age, sex distribution, verbal IQ, or parental SES. On average, FEP had fewer years of education, lower SES, markedly worse MATRICS neuropsychological performance than HC, and worse GFS scores, consistent with the debilitating effects of emerging psychosis on cognition and real world functioning (Table 1).
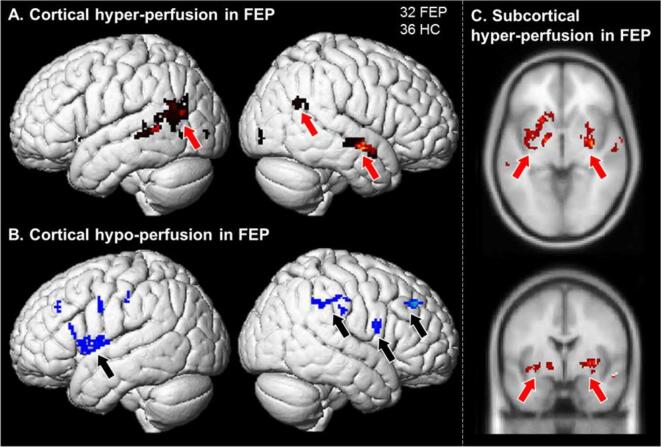
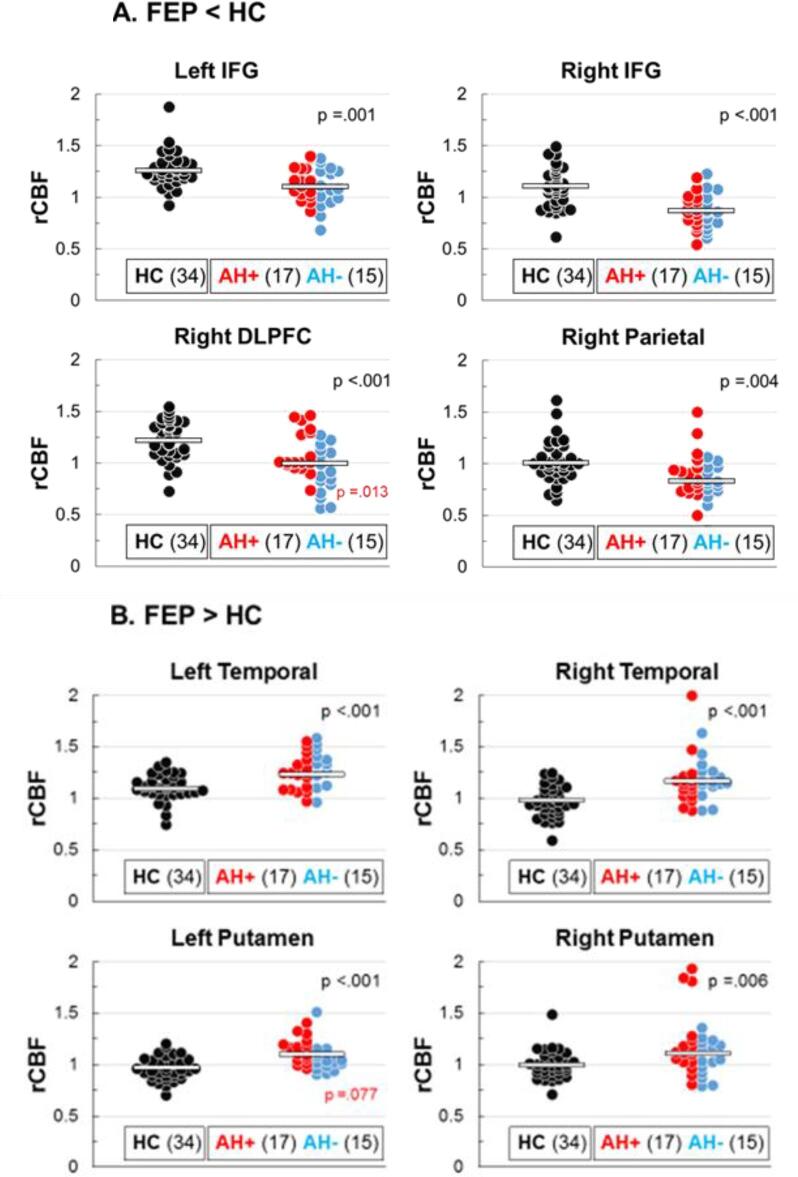
Whole brain data-driven TFCE comparing resting rCBF between FEP and HC identified eight clusters of significant differences (Fig. 2). Reduced rCBF in FEP was found in executive control and attention-related cortical areas, including the left inferior frontal gyrus (IFG) and frontal operculum (182 voxels), right IFG (33 voxels), the right dorsolateral prefrontal cortex (DLPFC; 37 voxels), and the right superior parietal cortex (89 voxels). Conversely, increased rCBF in FEP was found in left temporoparietal cortex, including Wernicke’s area (214 voxels), right temporal cortex (122 voxels), and left (355 voxels) and right putamen (465 voxels). Average rCBF within each of these regions was also compared between FEP who endorsed experiencing AH during clinical interviews (AH+, 3 or more on the PANSS, N = 17) and those who did not (AH−, 1 or 2 on the PANSS, N = 15). AH+ showed significantly greater activity in right DLPFC and marginally in left putamen relative to AH− individuals. Distributions of all participants, including AH+ and AH− subgroups, are presented in Fig. 3, with associated between group t-test statistics.
Fig. 2.
Whole brain analysis of pcASL revealed: A. Increased basal activity in left and right temporal-parietal areas and in right anterior STS auditory cortex; B. Reduced basal activity in right and left IFG, and right parietal executive control areas; and C. Increased basal activity in left and right putamen, with a role in semantic access.
Fig. 3.
Distribution of rCBF between HC and FEP. A. Areas where FEP shoed hypo-perfusion. B. Areas where FEP showed hyper-perfusion. FEP are further separated into hallucinators (AH+, red) and non-hallucinators (AH−,blue). Pathophysiology was generally similar between FEP AH subgruops. AH+ showed a bimodal distribution in right DLPFC, while AH+ were uniformly increased in left putamen comapered with AH−. Note: Black p values indicate HC vs FEP comparisons and red p values indicate AH+ vs AH− FEP comparisons, and red p valued indicate AH+ vs AH− FEP subgroup comparisions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
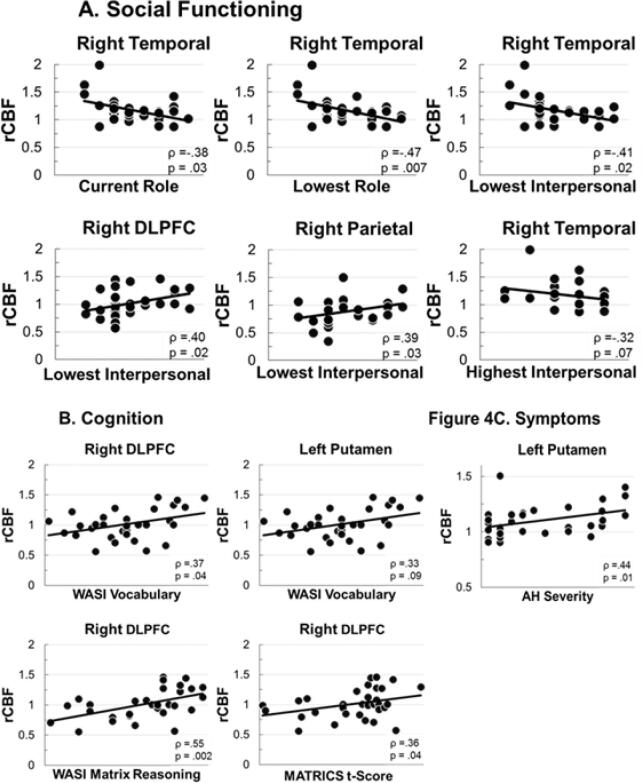
Within FEP, associations between cognitive functioning, social functioning, and symptom severity were assessed using Spearman’s rank-order correlations (Fig. 4). Among cognitive measures (Fig. 4A), WASI Vocabulary and Matrix Reasoning t-scores, and MATRICS overall composite t-scores were positively correlated with right DLPFC basal perfusion. Among social functioning measures (Fig. 4B), lower activity in the right temporal cluster was significantly associated with higher current role (occupational), higher worst role functioning levels in the last year (i.e., better function, even at the lowest point), and higher worst social (interpersonal) functioning in the last year, and at trend-level with better highest interpersonal functioning in the last year. In addition, higher values for lowest social (interpersonal) functioning in the last year were associated with higher right DLPFC and right parietal basal perfusion levels. Finally, among symptoms, no PANSS factor correlated significantly with any region. For AH SAPS component severity, left putamen overactivity was associated with greater hallucinations (Fig. 4C).
Fig. 4.
Significant and trend-level correlations between altered rCBF and: A. Social functioning; B. Cognition; and C. Auditory hallucinations.
5. Discussion
Eight areas were identified in a whole brain analysis of pathological perfusion in FEP. Six areas (left and right IFG, left temporal parietal area, right temporal cortex, and left and right putamen) belong to the language/auditory circuit implicated in fMRI studies and our DSI white matter study, and comprise nodes in our AH model (Fig. 1). Thus, nodes of the auditory/language circuit show pathological activity even at first psychotic episode. The right DLPFC and right posterior parietal areas belong to cognitive control areas and have been implicated in many fMRI studies of schizophrenia and psychosis, typically with respect to working memory (Carter et al., 1998, Cannon et al., 2005, Barch and Csernansky, 2007) but are not typically included in models of AH. A notable exception is the SYNOPSIS model of Strik and colleagues (Strik et al., 2017) that includes projections to the right parietal cortex in the communication circuit. Reduced basal perfusion in these areas (and left IFG in the language circuit) indicates pervasive reduction of hub areas related to cognitive control and differential inhibition of sensory/semantic activity in service of goal-directed activity.
Our hypothesis that pathological basal activity in the language/auditory circuit would correlate with AH severity was only partially supported, as increased activity in left putamen was associated with worse AH (Fig. 4C). Further, left putamen showed a trend for increased activity in AH+ compared to AH− (Fig. 3B). It is important to note that hyper- and hypo-activity were not associated with AH in remaining areas. Inspection of Fig. 3 reveals that AH+ and AH− showed essentially equal pathophysiology in most areas. This implies that loss of left and right IFG control and hyper-activation of left TPJ and right temporal cortex within the language/auditory circuit are associated more broadly with the emergence of psychosis, and are not exclusively associated with AH per se. In fact, right auditory cortex hyper-activity was robustly associated with poor social functioning, whereas pathological basal activity in cognitive control areas showed marked associations with lowest levels of interpersonal functioning in the year preceding first clinical contact. Furthermore, right DLPFC perfusion was associated with premorbid and current intellect, with more normative DLPFC activity correlated with better cognition (Fig. 4B). Interestingly, within the language/auditory circuit nodes, increased left putamen activity was associated with better WASI vocabulary performance (within the context of also being associated with more severe AH). Thus, at the first-episode of psychosis, reductions of basal perfusion in prefrontal control (left and right IFG, right DLPFC) and right parietal control areas are present, as are increases of basal perfusion in the posterior and subcortical components of the language/auditory processing circuitry. Associations with AH severity were not marked, and AH− FEP were generally as impaired in each area as AH+ individuals. These results suggest that metabolic dysregulation in these areas is more associated with psychosis in general. Future work will try to find associations with specific symptoms and behaviors, but in this sample, associations were at the broader level of psychosis itself.
Based on the pcASL perfusion findings of this report and altered transcallosal auditory cortex white matter connectivity (Salisbury et al., 2021), we present a revised model of systems-level pathophysiology in FEP (Fig. 5). Impaired sensory processing and interhemispheric communication provide weak perceptual objects for higher-order cognition. Lack of top-down auditory executive control and cognitive control lead to failure down-modulate spurious sensory and multimodal activation. The semantic system becomes dysregulated, with spurious activations that reflect memory intrusions. This system-level dysregulation affects symptoms, cognition, and social functioning, with the pervasive impairment evident as psychosis emerges.
Fig. 5.
Revised model of systems-level pathophysiology in FEP. In addition to the bottom up and executive semantic/auditory deficits decribed Fig. 1, general purpose executive control areas (right DLPFC and parietal cortex) show reduced activity. Each node within this circuitry presents a potential target for non-invasive brain stimulation.
Structural deficits in gray matter volume and thickness are present in left and right temporal areas at first break (e.g., Hirayasu et al., 2000), and although white matter anisotropy measures have been associated with AH at first break (See Salisbury et al., 2021 for review), the effect sizes are much smaller than for the elevated and reduced basal rCBF observed in this sample of FEP individuals. This is consistent with a functional systems-level deficit that may be amenable to biological intervention. Because this was a data-driven whole brain analysis of basal perfusion, areas identified were not selected a priori based on our hypothesis of aberrant language system physiology and psychosis emergence. Still, the language circuit and bilateral auditory executive IFG areas, and right hemisphere cognitive control areas showed marked abnormalities in FEP. These areas are of primary importance to the emergence of psychosis, and because the functional imbalance is not necessarily associated with irreversible structural pathology, our findings provide a key novel system for targeted intervention to ameliorate the debilitating course of psychosis. Non-invasive brain stimulation (NIBS) is becoming an increasingly explored treatment modality in psychiatry, termed interventional psychiatry. Techniques include regional transcranial magnetic stimulation (rTMS), low-field magnetic stimulation (LFMS), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS). Generally, these methods act by modulating the resting membrane potentials of targeted areas, causing relative de- or hyper-polarization of patches of cortex. Our findings suggest that targeted intervention to increase prefrontal and right parietal cognitive control activity and decrease left TPJ and right temporal activity may be a viable treatment in new psychosis. Deep brain TMS (dTMS) or, invasively, deep brain stimulation (DBS) implants targeting left putamen may be beneficial for intractable and debilitating AH.
Although substantial for a single-center study of FEP, the sample sizes reported remain small. Future testing of FEP in this center and in collaboration with others is needed to provide replication and refinement of these initial findings. The progressive nature of these basal perfusion abnormalities is unknown. Currently we are testing participants longitudinally to track and changes and associations between perfusion patterns and clinical outcomes. Testing participants longitudinally during the early course of psychosis is critical to determining if worsening or improvement of this systems-level imbalance is normalized with treatment and reflected in improved clinical course, or whether the pattern worsens inevitably with disease duration. The inter-relationship between brain function, structure, and symptoms needs to be determined longitudinally to plot how pathophysiology of the language and cognitive control circuits are associated with neural changes, and impact clinical manifestations.
In summary, pervasive hypo-activity of executive control areas and hyper-activity of auditory and semantic access areas of the brain were present broadly in FEP. Pathology in some areas was related to AH severity, but was pathological in others independently from AH status and severity. Right frontal and left and right temporal areas show a lack of reciprocal modulation that leads to metabolic imbalance in FEP. These cortical areas are geometrically situated for targeted NIBS that may ameliorate imbalance and restore healthy brain function in emerging psychosis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the staff of the Western Psychiatric Hospital Psychosis Recruitment and Assessment Core (Debra Montrose, Kevin Eklund, Alicia Thomas, Gretchen Haas, and Elizabeth Radomsky) for subject recruitment and assessment, and Karol Rosengarth for administrative support and quality assurance. As well, we thank all the subjects for participating.
Funding
Supported by R01 MH113533 to DFS.
Role of funding source
NIH played no role in the conducting or reporting of the project.
Data availability
Data will be made available on request.
References
- Addington J., Liu L., Buchy L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Bearden C.E., Mathalon D.H., McGlashan T.H. North American Prodrome Longitudinal Study (NAPLS 2) The Journal of Nervous and Mental Disease. 2015;203:328–335. doi: 10.1097/NMD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J., Wang D.J., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance Medicine. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J., Wang D.J., Wong E.C., Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance Medicine. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Csernansky J.G. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domain-general executive dysfunction. American Journal of Psychiatry. 2007;164:1090–1098. doi: 10.1176/ajp.2007.164.7.1090. [DOI] [PubMed] [Google Scholar]
- Cannon T.D., Glahn D.C., Kim J., Van Erp T.G., Karlsgodt K., Cohen M.S., Nuechterlein K.H., Bava S., Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of General Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Perlstein W., Ganguli R., Brar J., Mintun M., Cohen J.D. Functional hypofrontality and working memory dysfunction in schizophrenia. American Journal of Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Caspi, A., Houts, R.M., Belsky, D.W., Goldman-Mellor, S.J., Harrington, H., Israel, S., Meier, M.H., Ramrakha, S., Shalev, I., Poulton, R., & Moffitt, T.E. (2014). The p factor one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2:119-137. PubMed. [DOI] [PMC free article] [PubMed]
- Cornblatt B.A., Auther A.M., Niendam T., Smith C.W., Zinberg J., Bearden C.E., Cannon T.D. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T., Linden D.E., Jandl M., Formisano E., Goebel R., Lanfermann H., Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dolui S., Wang Z., Shinohara R.T., Wolk D.A., Detre J.A., Initiative A.D.N. Structural Correlation-based Outlier Rejection (SCORE) algorithm for arterial spin labeling time series. Journal of Magnetic Resonance Imaging. 2017;45:1786–1797. doi: 10.1002/jmri.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y., McCarley R.W., Salisbury D.F., Tanaka S., Kwon J.S., Frumin M., Snyderman D., Yurgelun-Todd D., Kikinis R., Jolesz F.A., Shenton M.E. Planum temporale and Heschl’s gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Archives of General Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Boutros N.N., Hu S., Berman R.M., Krystal J.H., Charney D.S. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. The Lancet. 2000;355:1073–1075. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Hawkins K.A., Gueorguieva R., Boutros N.N., Rachid F., Carroll K., Krystal J.H. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Archives of General Psychiatry. 2003;60:49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Hampson M., Wu K., Anderson A.W., Gore J.C., Buchanan R.J., Constable R.T., Hawkins K.A., Sahay N., Krystal J.H. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cerebral Cortex. 2007;17:2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R.E., Fernandez T., Pittman B., Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biological Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University; 1975. Four factor index of social status. [Google Scholar]
- Kindler J., Homan P., Jann K., Federspiel A., Flury R., Hauf M., Strik W., Dierks T., Hubl D. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biological Psychiatry. 2013;73:518–524. doi: 10.1016/j.biopsych.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Kotov R., Chang S.W., Fochtmann L.J., Mojtabai R., Carlson G.A., Sedler M.J., Bromet E.J. Schizophrenia in the internalizing-externalizing framework: a third dimension? Schizophrenia Bulletin. 2011;37:1168–1178. doi: 10.1093/schbul/sbq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox B.R., Park S.B.G., Medley I., Morris P.G., Jones P.B. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Research: Neuroimaging. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Longenecker J.M., Haas G.L., Salisbury D.F. Hierarchical Symptom Components in Early Psychosis. Schizophrenia Bulletin. 2022 doi: 10.1093/schbul/sbac048. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón J.V., Coupé P., Martí-Bonmatí L., Collins D.L., Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. Journal of Magnetic Resonance Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S., Smailes D., Corvin A., Gill M., Morris D.W., Dinan T.G., Murphy K.C., Waddington J.L., Bank A.S.R., Donohoe G., Dudley R. Occurrence and co-occurrence of hallucinations by modality in schizophrenia-spectrum disorders. Psychiatry Research. 2017;252:154–160. doi: 10.1016/j.psychres.2017.01.102. [DOI] [PubMed] [Google Scholar]
- Mueser K.T., Bellack A.S., Brady E.U. Hallucinations in schizophrenia. Acta Psychiatrica Scandinavica. 1990;82:26–29. doi: 10.1111/j.1600-0447.1990.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Salisbury D.F., Kohler J., Shenton M.E., McCarley R.W. Deficit effect sizes and correlations of auditory event-related potentials at first hospitalization in the schizophrenia spectrum. Clinical EEG and Neuroscience. 2020;51:198–206. doi: 10.1177/1550059419868115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury D.F., Wang Y., Yeh F.C., Coffman B.A. White matter microstructural abnormalities in the Broca’s-Wernicke’s-Putamen “Hoffman Hallucination Circuit” and auditory transcallosal fibers in first-episode psychosis with auditory hallucinations. Schizophrenia Bulletin. 2021;47:149–159. doi: 10.1093/schbul/sbaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik W., Dierks T., Hubl D., Horn H. Hallucinations, thought disorders, and the language domain in schizophrenia. Clinical EEG and Neuroscience. 2008;39:91–94. doi: 10.1177/155005940803900214. [DOI] [PubMed] [Google Scholar]
- Strik W., Stegmayer K., Walther S., Dierks T. Systems neuroscience of psychosis: mapping schizophrenia symptoms onto brain systems. Neuropsychobiology. 2017;75:100–116. doi: 10.1159/000485221. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Yuasa S., Minabe Y., Murata M., Kurachi M. Left superior temporal blood flow increases in schizophrenic and schizophreniform patients with auditory hallucination: A longitudinal case study using123I-IMP SPECT. European Archives of Psychiatry and Clinical Neuroscience. 1993;242:257–261. doi: 10.1007/BF02190383. [DOI] [PubMed] [Google Scholar]
- Toh W.L., Thomas N., Rossell S.L. Auditory verbal hallucinations in bipolar disorder (BD) and major depressive disorder (MDD): A systematic review. Journal of Affective Disorders. 2015;184:18–28. doi: 10.1016/j.jad.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Wang Z., Aguirre G.K., Rao H., Wang J., Fernandez-Seara M.A., Childress A.R., Detre J.A. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Manual for the Wechsler Abbreviated Intelligence Scale (WASI) [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N.D., Grön G., Sambataro F., Vasic N., Frasch K., Schmid M., Thomann P.A., Wolf R.C. Magnetic resonance perfusion imaging of auditory verbal hallucinations in patients with schizophrenia. Schizophrenia Research. 2012;134:285–287. doi: 10.1016/j.schres.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Wolf A.W., Schubert D.S., Patterson M.B., Grande T.P., Brocco K.J., Pendleton L. Associations among major psychiatric diagnoses. Journal of Consulting and Clinical Psychology. 1988;56:292–294. doi: 10.1037//0022-006x.56.2.292. [DOI] [PubMed] [Google Scholar]
- Wright A.G., Krueger R.F., Hobbs M.J., Markon K.E., Eaton N.R., Slade T. The structure of psychopathology: toward an expanded quantitative empirical model. Journal of Abnormal Psychology. 2013;122:281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.