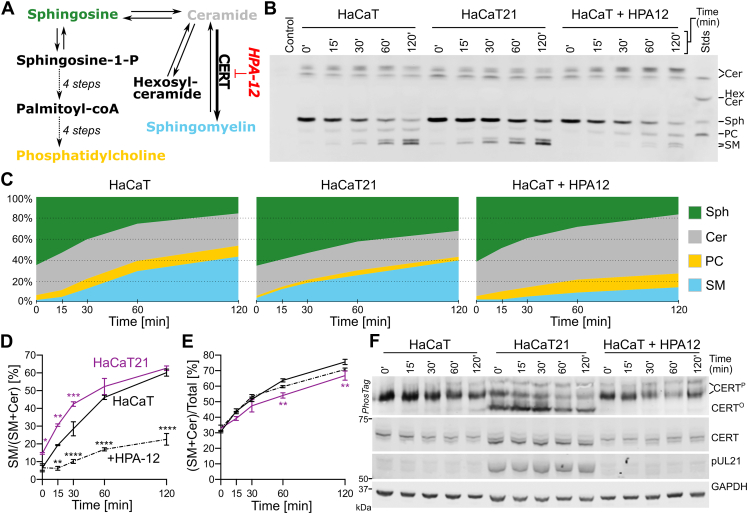
Figure 1.
Herpes simplex virus 1 (HSV-1) pUL21 alters the rate of ceramide (Cer) to sphingomyelin (SM) conversion in cultured cells.A, simplified schematic diagram of sphingolipid biosynthetic pathways that lead from sphingosine (Sph) to SM and glycosphingolipids (hexosylceramide; HexCer), or to phosphatidylcholine (PC) via a “salvage” pathway. In this salvage pathway, Sph-1-phosphate is converted to trans-2-hexadecenal, then trans-2-hexadecenoic acid and trans-2-hexadecenoyl-coA before being converted to palmitoyl-coA (112), which is in turn conjugated to glycerol-3-phosphate to form lysophosphatidic acid before being converted to phosphatidic acid, diacylglycerol, and then PC (113). Cer to SM conversion is accelerated by the transport protein CERT, which is selectively inhibited by HPA-12. B, rate of Sph conversion to Cer, SM, or PC was measured in HaCaT cells, untreated or treated with 1 μM HPA-12, and in HaCaT cells stably expressing pUL21 (HaCaT21). Cells were incubated with 5 μM “clickable” alkyne-Sph (pulse) for 5 min and harvested for lipid extraction either immediately (0 min) or at the indicated times (chase). Extracted lipids were bioconjugated to 3-azido-7-hydroxycoumarin, separated by HPTLC and detected using UV light. Separated lipids were identified using clickable standards (Stds) and previous literature (42). Data are from one representative experiment of two independent repeats. C, quantitation of the lipid intensities from (B) as determined by densitometry and represented as percentage fraction of total signal. D, rate of SM synthesis expressed as its fraction in the cumulative signal for SM and Cer. E, the ratio of alkyne-Sph incorporated into either Cer or SM as a proportion of total alkyne-lipid signal, representing the influx of Sph into the SM biosynthetic pathway. For D and E, the data represent two independent experiments (mean ± SD). Data points are labeled if significantly different to parental HaCaT cells: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; and ∗∗∗∗p < 0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test). F, the resolubilized proteins precipitated during lipid extraction were analyzed by SDS-PAGE and immunoblotting using the antibodies listed. Where indicated, the gel was supplemented with PhosTag reagent to retard the migration of phosphorylated proteins, thus enhancing the separation of CERT that is hypophosphorylated (CERTO) or hyperphosphorylated (CERTP). GAPDH serves as a loading control.