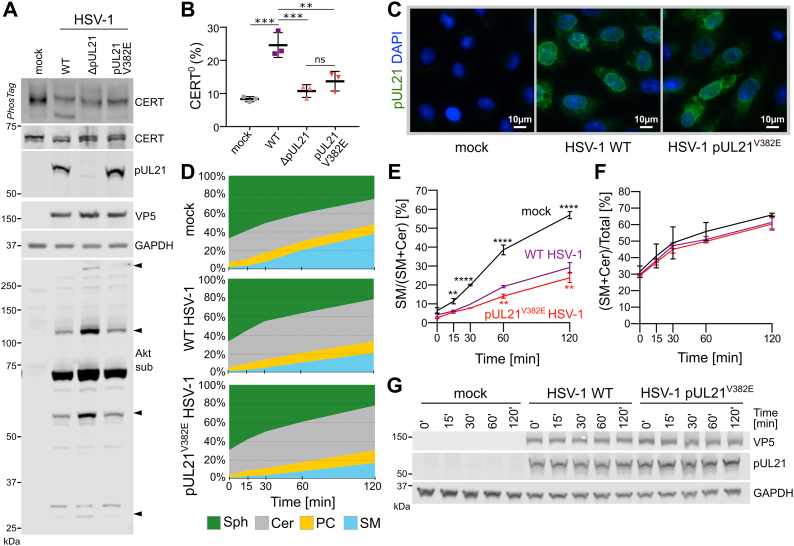
Figure 5.
Mutating the CERT-binding interface of pUL21 inhibits CERT dephosphorylation and reduces the rate of sphingomyelin synthesis in infected cells.A, HaCaT cells were infected at MOI = 5 with WT HSV-1, HSV-1 lacking pUL21 expression (ΔpUL21), or a pUL21 point mutant virus (pUL21V382E). Lysates were harvested at 16 hpi in the presence of phosphatase inhibitors and subjected to SDS-PAGE plus immunoblotting using the antibodies listed. Where indicated, the gel was supplemented with PhosTag reagent to enhance separation of CERT phosphoforms. The antibody recognizing phosphorylated Akt substrates (Akt sub) illustrates activity of the HSV-1 kinase pUS3, several substrates of which are dephosphorylated in a pUL21-dependent manner (arrowheads) (18). VP5, infection control. B, quantitation of the CERT dephosphorylation level (ratio of CERTO to total CERT) in cells infected with WT or mutant HSV-1, as determined by densitometry. Results are presented as mean ± SD from three independent experiments. One-way ANOVA with Tukey’s multiple comparisons test was used for the statistical analysis (ns, nonsignificant; ∗∗p < 0.01; ∗∗∗p < 0.001). C, Vero cells were infected at MOI = 1, fixed at 14 hpi, and stained with DAPI (blue) plus an antibody recognizing pUL21 (green). D, pulse-chase experiment to measure the rate of Sph conversion to Cer, SM, and PC. HaCaT cells were infected with WT or pUL21V382E HSV-1 at MOI = 5 or mock infected. Cells were incubated with 5 μM alkyne-Sph (pulse) at 14 hpi for 5 min and harvested for lipid extraction either immediately (0 min) or at the indicated times (chase). Extracted lipids were bioconjugated to 3-azido-7-hydroxycoumarin using click chemistry, separated by HPTLC, visualized using UV light, and relative lipid abundances were quantitated by densitometry. Data are from one representative experiment of two independent repeats. E, rate of SM synthesis expressed as its fraction in the cumulative signal for SM and Cer. F, the proportion of alkyne-Sph incorporated into either Cer or SM, representing the temporal influx of Sph into the SM biosynthesis pathway. E and F, the data represent two independent experiments (mean ± SD). Data points are labeled if significantly different to WT HSV-1: ∗∗p < 0.01; ∗∗∗∗p < 0.0001 (two-way ANOVA with Dunnett’s multiple comparisons test). G, the resolubilized proteins precipitated during lipid extraction were analyzed by SDS-PAGE and immunoblotting using the antibodies listed. Cer, ceramide; DAPI, 4′,6-diamidino-2-phenylindole; hpi, hours postinfection; HPTLC, high-performance TLC; HSV-1, herpes simplex virus 1; MOI, multiplicity of infection; PC, phosphatidylcholine; SM, sphingomyelin; Sph, sphingosine.