Figure 2 |. Mechanisms, synthesis and kinetics.
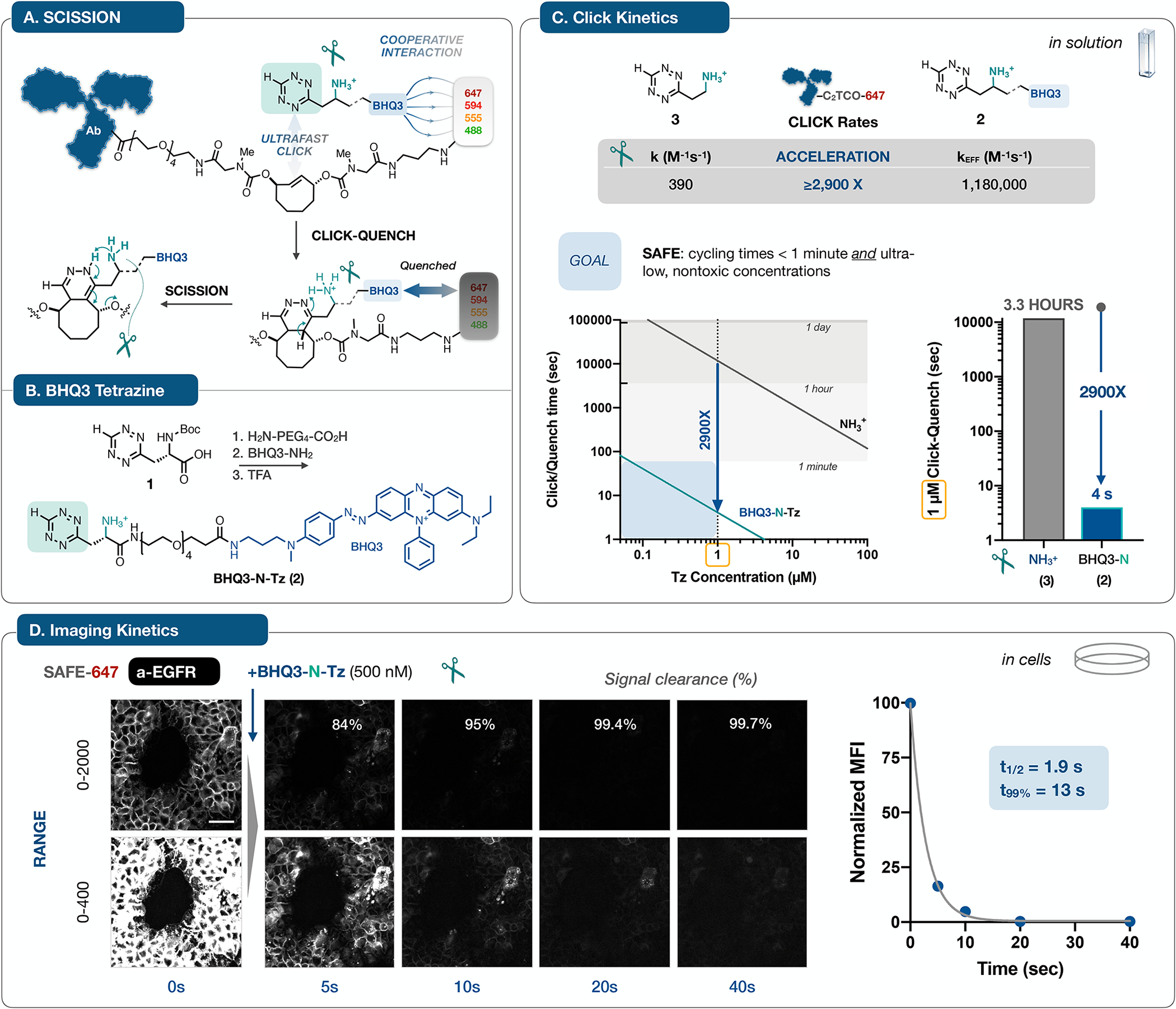
A. Antibodies labeled with C2TCO-linked dyes are rendered non-fluorescent in a concerted two step sequence: noncovalent interactions between BHQ3 and the fluorophore can accelerate the Tz/TCO click reaction33, leading to immediate quenching upon ligation; in turn, the functionalized ‘Tz-scissors’ drives instantaneous tautomerization and scission of the dye. The design of C2TCO itself ensures that the cleavage reaction proceeds irrespective of the Tz/TCO click orientation. B. Starting from the Boc-protected Tz (1), a hydrophilic PEG linker and BHQ3 are conjugated and then deprotected to yield BHQ3-N-Tz (2). C. Quantification of reaction kinetics for the click reaction of 2 with an AF647-C2TCO-labeled antibody in solution revealed an effective rate constant of >106, a marked ~3,000-fold acceleration relative to the parent aminoethyl-functionalized Tz (3)37. This acceleration shifts the concentration/time relationship into the needed range for efficient cycling at ≤ 1 μM reagent concentrations. D. To validate the accelerated kinetics in the cellular context, we collected time lapse images of A431 cells stained with a SAFE647-anti-EGFR antibody and then treated with BHQ3-N-Tz (2). Images rendered at two window levels capture the rapid (t1/2 = 1.9 s, one phase decay) and quantitative clearance of the fluorophore signal, reaching 99.7% removal within 40 seconds, concordant with the in vitro assay result. See Supplementary Fig. 3 for analytical details. Normalized mean fluorescence intensity (MFI) is plotted as mean ± SD; error bars are smaller than the symbol size). The scale bar is 50 μm
