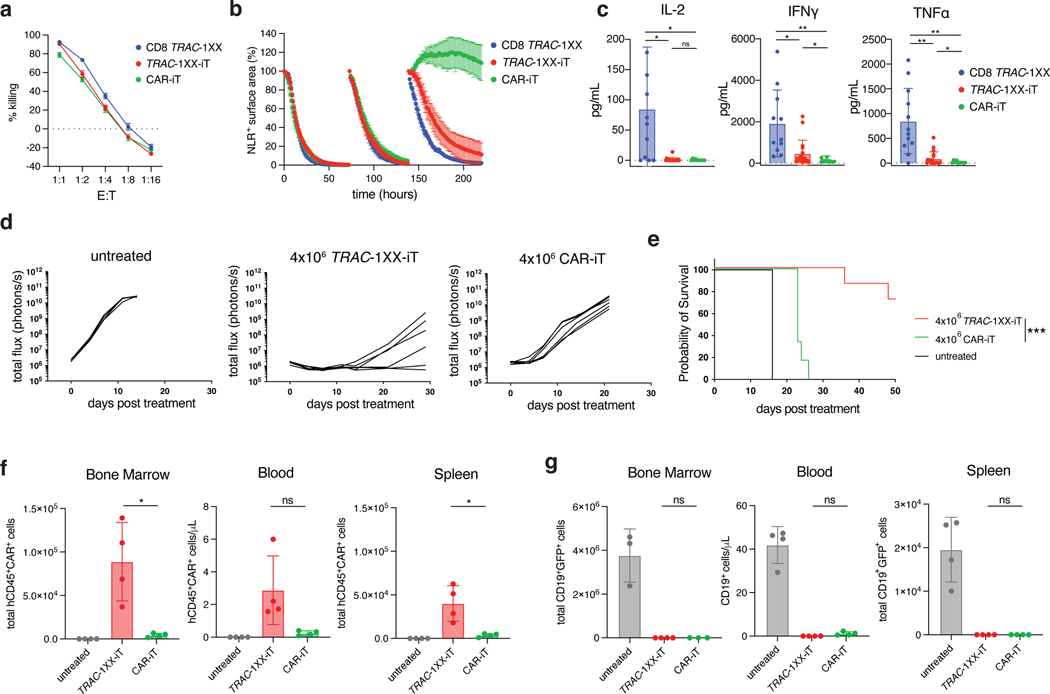
Fig. 6. TRAC-1XX-iT have improved persistence and function over CAR-iT cells.
Functional comparison of healthy-donor peripheral blood TRAC-1XX CD8αβ αβTCR-T cells (CD8 TRAC-1XX), CAR-iT, and TRAC-1XX-iT cells (matured on 3T3-CD19–41BBL). CD8 TRAC-1XX doses reflect number of CAR+ T cells utilized in the assay. a, Cytotoxic activity using a 18 h Incucyte assay, using NLR-expressing NALM6 as target cells (n = 3 technical replicates). b, NALM6 rechallenge assay. NLR+ NALM6 and T cells were co-cultured at a 1:1 E:T. Every 72 h T cells were rechallenged with 1x NLR+NALM6 and cytokines. NALM6 clearance was measured in NLR+ surface area reduction compared to the timepoint of rechallenge (n = 3 technical replicates). c, Twenty-four h cytokine secretion using NALM6 as target cells at a 1:1 E:T ratio (CD8 TRAC-1XX n = 15, TRAC-1XX-iT n = 18, CAR-iT n = 11 biological replicates, IL-2 CD8 TRAC-1XX vs CAR-iT p=0.02, IL-2 CD8 TRAC-1XX vs TRAC-1XX-iT p=0.0222, IFNγ CD8 TRAC-1XX vs CAR-iT p=0.004, IFNγ CD8 TRAC-1XX vs TRAC-1XX-iT p=0.0293, IFNγ TRAC-1XX-iT vs CAR-iT p=0.0363, TNFα CD8 TRAC-1XX vs CAR-iT p=0.001, TNFα CD8 TRAC-1XX vs TRAC-1XX-iT p=0.0027, TNFα TRAC-1XX-iT vs CAR-iT p=0.0262). d, Tumour burden (total flux in photons per second) of NALM6-bearing, untreated mice (n = 4) or mice treated with 4×106 TRAC-1XX-iT (middle) or CAR-iT (right) cells (n = 6, line = one mouse). e, Kaplan-Meier analysis of overall survival (p=0.002). f,g, Enumeration of iT cells (f) and tumour cells (g) in the bone marrow, spleen and blood 12 days post T cell infusion (n = 4 mice, iT in bone marrow TRAC-1XX-iT vs CAR-iT p=0.0329, iT in spleen TRAC-1XX-iT vs CAR-iT p=0.0369). * P<0.05, ** P<0.01, *** P<0.001, Welch’s 2-sample two-sided t test (c, f, g), log-rank Mantel-Cox test (e). All data are means ± s.d.