Figure 3:
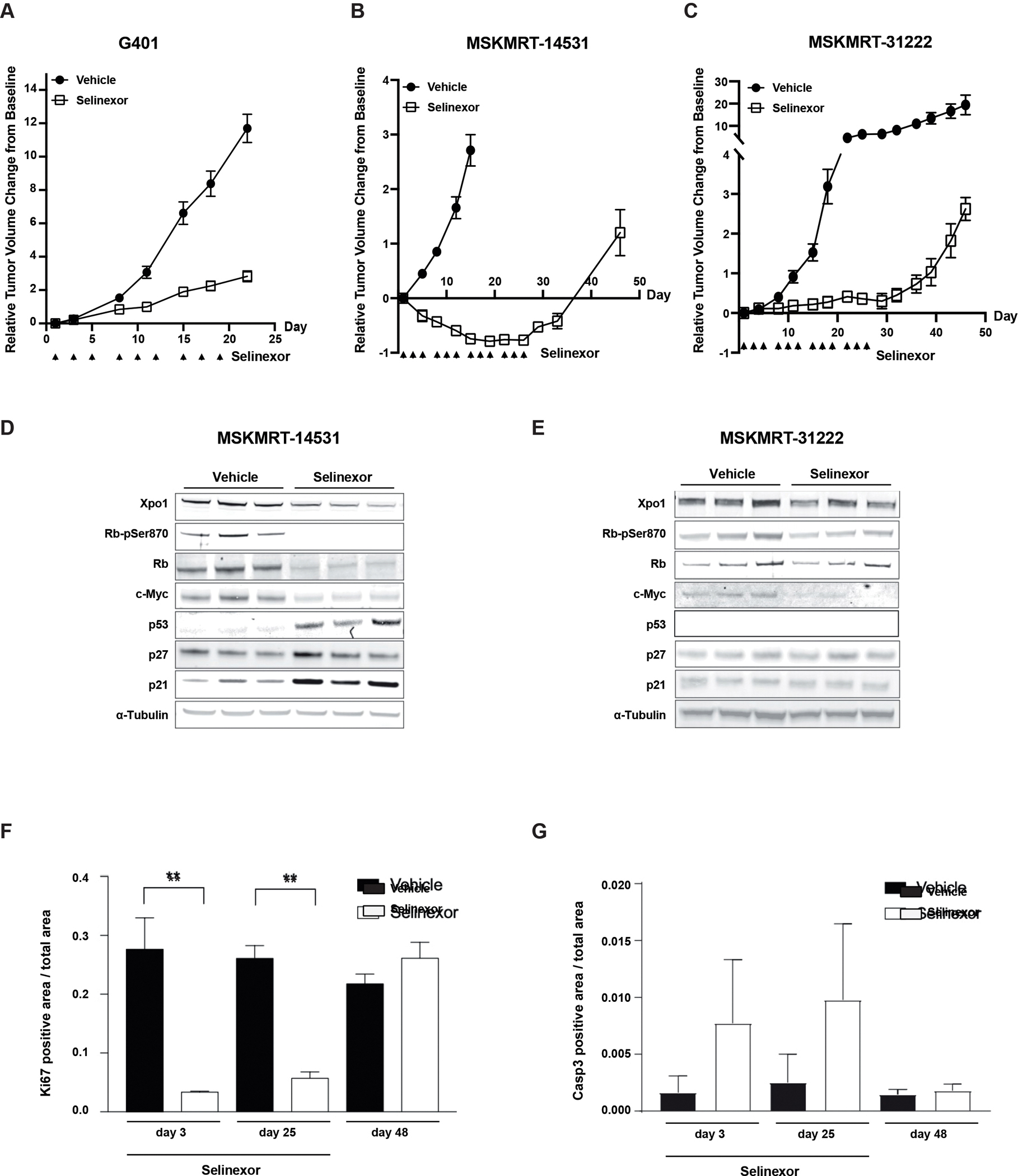
In vivo activity of selinexor in MRT xenografts. A) Tumor response in G401 xenograft (n= 8 per arm) treated with vehicle or selinexor for 21 days. Error bars: standard error of mean (SEM). B) and C) Tumor responses in MRT patient-derived xenograft (PDX) models (n=8/arm) treated with selinexor for 28 days. Error bars: SEM. D-E) Pharmacodynamic assessment of XPO1 and proliferative markers in MRT PDX models treated with selinexor. F) Immunohistochemical analysis of proliferation (Ki67) and G) apoptosis induction (caspase 3) in an MRT PDX model treated with selinexor (through Day 25) and following withdrawal of treatment.
