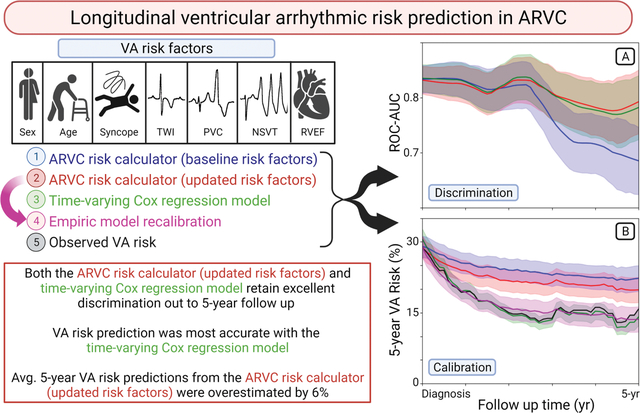
Abstract
Background:
The arrhythmogenic right ventricular cardiomyopathy (ARVC) risk calculator stratifies risk for incident sustained ventricular arrhythmias (VA) at the time of ARVC diagnosis. However, included risk factors change over time, and how well the ARVC risk calculator performs at follow-up is unknown.
Methods:
This was a retrospective analysis of patients with definite ARVC and without prior sustained VA. Risk factors for VA including age, non-sustained ventricular tachycardia, premature ventricular complex burden, T-wave inversions on electrocardiogram, cardiac syncope, right ventricular function, therapeutic medication use, and exercise intensity were assessed at the time of 2010 Task Force Criteria based ARVC diagnosis and upon repeat evaluations. Changes in these risk factors were analyzed over 5-year follow-up. The 5-year risk of VA was predicted longitudinally using 1) the baseline ARVC risk calculator prediction, 2) the ARVC risk prediction calculated using updated risk factors, and 3) time-varying Cox regression. Discrimination and calibration were assessed in comparison to observed VA event rates.
Results:
408 ARVC patients experiencing 132 primary VA events were included. Matched comparison of risk factors at baseline versus at 5-years of follow-up revealed decreased burdens of premature ventricular complexes (−1,200/day) and non-sustained ventricular tachycardia (−14%). Presence of significant right ventricular dysfunction and number of T-wave inversions on electrocardiogram were unchanged. Observed risk for VA decreased by 13% by 5-years follow-up. The baseline ARVC risk calculator’s ability to predict 5-year VA risk worsened during follow-up (c-statistic, 0.83 at diagnosis versus 0.68 at 5-years). Both the updated ARVC risk calculator (c-statistics of 0.77) and time-varying Cox regression model (c-statistic 0.77) had strong discrimination. The updated ARVC risk calculator overestimated 5-year VA risk by an average of +6%.
Conclusion:
Risk factors for VA in ARVC are dynamic, and overall risk for incident sustained VA decreases during follow-up. Up-to-date risk factor assessment improves VA risk stratification.
Keywords: Arrhythmogenic cardiomyopathy, clinical predictive models, ventricular tachycardia, risk stratification, sudden cardiac arrest
Graphical Abstract
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC), the most common form of arrhythmogenic cardiomyopathy, is a heterogeneous genetic disease characterized by fibro-fatty infiltration of the myocardium and the development of potentially lethal ventricular arrhythmias (VA)1. While ARVC is rare with a prevalence of only 1 in 1,000 to 1 in 5,0002, 3, it accounts for 10% to 20% of sudden cardiac deaths (SCD) in young adults4. The judicious implantation of cardioverter defibrillators (ICDs) in high-risk patients with ARVC is thus a core component of disease management. However, device-related risks are well known and may be particularly impactful among ARVC patients who are generally diagnosed at younger ages5, 6. Prospectively identifying those patients who are at high risk for VA, and consequently more likely to derive benefit from ICD placement, is therefore of critical importance in implantation decision making.
The ARVC risk calculator was recently proposed as a tool for individualized VA risk assessment7. The Cox proportional hazards-based ARVC risk calculator incorporates a series of seven clinical predictors (age, sex, right ventricular ejection fraction (RVEF), premature ventricular complex (PVC) burden on ambulatory cardiac monitoring, history of non-sustained ventricular tachycardia (NSVT), the total number of T-wave inversions (TWI) in precordial and inferior leads on electrocardiogram (ECG), and history of recent cardiac syncope) to determine a particular patient’s likelihood of developing incident VA over the five year period following his or her ARVC diagnosis. This tool has demonstrated excellent ability to discriminate between low- and high-risk ARVC patients (c-statistic, of 0.77) and has been increasingly adopted into clinical ICD decision making algorithms8. However, ARVC is a progressive condition and clinical predictors included in the ARVC risk calculator may be dynamic9–11. How reliably this tool performs at subsequent evaluations after initial diagnosis is therefore unknown, limiting longitudinal risk assessment in these patients.
To address this question, we analyzed data from a large, multi-center cohort of ARVC patients without prior sustained VA which included repeat clinical, imaging, and electrophysiologic assessments during routine follow-up. We hypothesized that VA risk predictors change over time, and that incorporation of these changes is necessary for accurate longitudinal risk prediction in ARVC.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We conducted an observational, retrospective, longitudinal cohort study in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement12.
Study Population
The study population comprised patients enrolled in the Johns Hopkins ARVC registry and the Netherlands Arrhythmogenic Cardiomyopathy (ACM) registry. In brief, consecutive patients from were included in the current study if (i) they were diagnosed with definite ARVC by the 2010 Task Force Criteria1, (ii) had not experienced prior sustained VA at the time of ARVC diagnosis, and (iii) had longitudinal clinical follow-up of at least 1 day. This study conforms to the Helsinki declaration and was approved by local ethics and/or institutional review boards. Participants signed informed consent to have their data included in the registry.
Data Collection
As described previously7, data were collected independently by each participating center using uniform definitions (Supplemental Table I). Outcomes and baseline characteristics were adjudicated at each center via review of clinical visit documentation, ECG tracings, ICD interrogation tracings, ambulatory cardiac monitoring reports, echocardiography reports, cardiac magnetic resonance imaging (CMR) reports, as well as medical and death records. Genetic variants were adjudicated according to the American College of Medical Genetics and Genomics guidelines by consensus of specialists in cardiac genetics13. Additional longitudinal data from subsequent clinical follow-up were also collected, including repeat ECG tracings, echocardiography reports, ambulatory cardiac monitoring reports, prescribed medication reviews, and exercise histories. Due to high prevalence of ICD implantation in these patients and the resulting low number of repeat CMR studies performed during follow-up, longitudinal CMR data was not included.
Study Outcomes
Consistent with the published ARVC risk calculator7, the primary outcome was first sustained VA following confirmed ARVC diagnosis. Sustained VA was defined as a composite of SCD, sudden cardiac arrest, spontaneous sustained ventricular tachycardia [VT; lasting ≥30 seconds (s) at ≥100 beats per minute (bpm) or with hemodynamic compromise requiring cardioversion], ventricular fibrillation, or appropriate ICD intervention (defined as anti-tachycardia pacing or defibrillation). Incident heart transplantation, cardiovascular mortality, and all-cause mortality were also collected.
Predictor Variables and the ARVC Risk Calculator
Variables included in the ARVC risk calculator were considered7. These include sex, age, recent cardiac syncope (defined as transient loss of consciousness and postural tone with spontaneous recovery with a likely arrhythmic mechanism within the preceding 6-months), presence of NSVT (defined as hemodynamically stable VT at ≥100 bpm, for ≥3 beats <30 s), burden of PVCs on most recent 24-hour ambulatory cardiac monitoring, extent (defined as sum) of TWI on anterior and inferior leads on ECG, and RVEF. Each predictor variable was determined at the time of diagnosis, defined as within one year of ARVC diagnosis but always before arrhythmic outcome, and at each follow-up visit. The timing of clinical follow-up was based upon the discretion of local physicians.
Due to the limited availability of CMR-derived RVEF assessments during follow-up, the ARVC risk calculator was modified by replacing the RVEF variable with presence of moderate or severe RV dysfunction as a dichotomous, echocardiographically-derived variable (Supplemental Methods I). We will refer to this model as the “modified ARVC risk calculator”, the formula of which is presented in Equation 1,
| (1) |
where prognostic index is calculated according to Equation 2.
| (2) |
Discrimination of this model was assessed using concordance-based c-statistic and 5-fold cross validation.
Longitudinal VA Risk. Risk Predictor Trends, and Risk Prediction at Follow-up
Longitudinal trends in risk factors included in the ARVC risk calculator, as well as those of alternative risk modifiers (left ventricular ejection fraction (LVEF), prescription rates of anti-arrhythmic medications and beta blockers, and level of athletic activity) were assessed by plotting the change in risk factor values relative to their patient-matched value at the time of diagnosis as a function of follow-up time. A window size equal to 2 years was used for the moving average, and analysis was limited to those patients for whom relevant testing/evaluation was available at both time of diagnosis and at follow-up. Patient data was not censored by VA event.
Longitudinal VA risk was estimated by repeating Kaplan-Meier analysis at each follow-up time out to 5-years. Patients were included in these analyses if they remained free of VA at the assessed follow-up time. Longitudinal prediction of 5-year VA risk was performed using three methods for interval follow-up risk estimation:
Baseline ARVC risk calculator: risk prediction calculated using only risk factors available at the time of diagnosis.
Updated ARVC risk calculator: risk prediction calculated using the most recent set of risk factors available at the time of follow-up evaluation.
Time-varying Cox regression: non-proportional Cox regression model that predicts risk as a function of both changing risk factors and the baseline hazard function (Supplemental Methods II).
Statistical analysis
Analyses were performed using PyCharm software version 2021.2 (JetBrains Inc., Boston, MA, USA) and open-source Pandas data analysis library, Lifelines survival analysis library, and statsmodels statistical modeling library. Missingness in data for the predictors included in the baseline ARVC calculator was assumed to be at random and imputed using multiple imputation with chained equations14. The final model included all predictors included in the ARVC risk calculator together with VA outcome and a cumulative baseline hazard estimation. A total of 20 imputed datasets were generated using 20 iterations each, and the final estimates were combined using Rubin’s rule15. Categorical variables were summarized as frequencies (%) and compared using proportional z-test. Continuous variables were presented as mean ± standard deviation or median [interquartile range (IQR)], and compared using the independent sample Students t-test or the Mann–Whitney U-test, as appropriate.
For patients with known risk factor values at both the time of diagnosis and at least 3-years of follow-up, Wilcoxon signed rank tests were used to assess differences between risk factor values at the two time points. For patients with >1 repeat risk factor assessment, the value from closest to 5-years of follow-up was selected. Follow-up duration was calculated from the date of diagnosis to the date of composite outcome occurrence or censoring (defined as death from any other cause, heart transplantation, or the most recent follow-up visit). Survival curves were estimated using the Kaplan–Meier method. The strengths of associations between risk factor variables and VA events were reported as hazard ratios derived from Cox proportional hazards modeling of baseline risk factors and from unrestricted Cox regression analysis of all available longitudinal risk factor data.
The longitudinal performances of the three methods for estimating 5-year VA risk were compared by generating time-dependent receiver operator characteristics and calculating the area under these curves (ROC-AUC) for each follow-up time between time of diagnosis and 5-years 16; error was reported with 95% confidence intervals and curves were smoothed to facilitate visual interpretation using locally weighted scatterplot smoothing with a weighting fraction of 0.2. Calibration was assessed by calculating the mean risk predictions for low (0–10%), intermediate (10–25%), and high (>25%) risk patients as assessed by the modified ARVC risk calculator at time of diagnosis, and comparing to mean observed risk as estimated by the Kaplan-Meier method in these risk groups. Differences in predicted versus observed risk (miscalibration) were assessed using empiric exponential decay functions (Supplemental Methods III) for both the overall cohort and each of the three risk groups.
Results
Study Population
The study included 408 patients, of whom 146 were from the Netherlands ACM registry (36%) and 262 were from the Johns Hopkins ARVC registry (64%). Patient characteristics by registry are shown in Supplemental Table II. A minority were male (n=164, 40%). The age at ARVC diagnosis was 37±15 years and about two-thirds had symptoms attributable to ARVC at the time of diagnosis (n=232, 64%). Most patients were identified as having pathogenic genetic variants (n=298, 74%), most commonly in PKP2 (n=197, 49%). More than half the patients were probands (n=240, 58.8%). Table 1 summarizes other clinical and demographic characteristics.
Table 1:
Baseline characteristics of ARVC patients at the time of ARVC diagnosis. Continuous variables are presented as mean ± standard deviation or median [IQR], as appropriate.
| Variable (Patients with available data at diagnosis) | All Patients (n=408) | Absence of VA event (276) | Occurrence of VA event (132) | p value |
|---|---|---|---|---|
| Age at Diagnosis (n=408) | 37 (±15.1) | 38 (±15.8) | 33 (±12.6) | <0.001 |
| Male Sex (n=408) | 164 (40.2%) | 96 (34.8%) | 68 (51.5%) | 0.001 |
| Caucasian Race (n=407) | 397 (97.5%) | 269 (97.5%) | 128 (97.7%) | 0.881 |
| Proband (n=408) | 240 (58.8%) | 135 (48.9%) | 105 (79.5%) | <0.001 |
| Pathogenic / likely pathogenic variant (n=405) | 298 (73.6%) | 211 (77.0%) | 87 (66.4%) | 0.024 |
| PKP2 | 197 (48.6%) | 133 (48.5%) | 64 (48.9%) | 0.953 |
| DSP | 13 (3.2%) | 10 (3.6%) | 3 (2.3%) | 0.468 |
| DSG2 | 11 (2.7%) | 5 (1.8%) | 6 (4.6%) | 0.111 |
| PLN | 27 (6.7%) | 20 (7.3%) | 7 (5.3%) | 0.46 |
| Other | 13 (3.2%) | 7 (2.6%) | 6 (4.6%) | 0.279 |
| Symptoms* (n=361) | 232 (64.3%) | 131 (55.3%) | 101 (81.5%) | <0.001 |
| History of Cardiac Syncope (n=408) | 77 (18.9%) | 39 (14.1%) | 38 (28.8%) | <0.001 |
| Anterior T-wave inversions (n=398) | 3 [2.0; 4.0] | 3 [1.0; 4.0] | 3 [3.0; 4.0] | <0.001 |
| Inferior T-wave inversions (n=387) | 0 [0.0; 1.0] | 0 [0.0; 1.0] | 0 [0.0; 1.0] | 0.028 |
| Total T-wave inversions (ant.+inf.) (n=387) | 3 [2.0; 5.0] | 3 [2.0; 4.0] | 4 [3.0; 5.0] | <0.001 |
| 24 hr. PVC count (n=343) | 1186 [361; 4095] | 860 [183; 2751] | 2879 [1151; 60785] | <0.001 |
| Presence of NSVT (n=370) | 195 (52.7%) | 114 (44.4%) | 81 (71.7%) | <0.001 |
| RVEF (%) (n=348) | 44 (±10.1) | 45 (±8.8) | 40 (±11.6) | <0.001 |
| LVEF (%) (n=355) | 58 (±8.0) | 58 (±7.9) | 57 (±8.3) | 0.304 |
| ICD at any point (n=407) | 277 (68.1%) | 150 (54.5%) | 127 (96.2%) | <0.001 |
| ICD prior to dx. | 18 (4.4%) | 16 (5.8%) | 2 (1.5%) | 0.049 |
| ICD within 6 mo. of dx. | 129 (31.6%) | 59 (21.4%) | 70 (53.0%) | <0.001 |
| ICD arrhythmia monitoring zone cycle length (ms) | 350 [323, 400] | 350 [328, 375] | 351 [322, 400] | 0.520 |
| ICD arrhythmia treatment zone cycle length (ms) | 300 [286, 320] | 300 [285, 316] | 300 [289, 333] | 0.017 |
| Baseline anti-arrhythmic prescription (n=391) | 59 (15.1%) | 36 (13.5%) | 23 (18.4%) | 0.21 |
| Amiodarone prescription | 8 (2%) | 5 (2%) | 3 (2%) | 0.753 |
| Sotalol prescription | 45 (11%) | 27 (10%) | 18 (14%) | 0.245 |
| Baseline beta-blocker prescription (n=392) | 153 (39.0%) | 96 (36.0%) | 57 (45.6%) | 0.068 |
| ARVC calculator predicted 5-year VA risk (%) | 29 (±23%) | 21 (±19%) | 45 (±23%) | <0.001 |
| Observed 5-year VA risk (%) | 29% [95%CI: 24, 34] | --- | --- |
Overall, 282 patients (69%) had complete baseline risk factor data allowing for estimation of the ARVC calculator 5-year VA risk. Missing data occurred for five of the eight predictors: NSVT (n=38, 9.3%), PVC count (n=65, 15.9%), number of TWI (n=21, 5.1%), RVEF (n=60, 14.7%). After imputation, mean 5-year VA risk was estimated at 29% [95% CI: 24, 34%] using the ARVC risk calculator.
Outcomes
During median follow-up of 5.2 [IQR: 2.8, 9.6] years, 132 (32%) patients experienced the composite VA outcome at a rate of 6.3 events per 100 patient-years. Figure 1 shows the cumulative VA free survival. Events occurred throughout follow-up, with a cumulative VA free survival at 5-years of 71.3% [95% CI: 75.8, 66.1]. Of these events, 87 (66%) were ICD interventions, including either appropriate shock or anti-tachycardic pacing, and had median cycle length of 270 ms [IQR: 235, 300]. Rapid sustained VAs (VT with cycle length <240 ms, SCA, or SCD) occurred in 41 (10.0%) patients during follow-up at a rate of 1.6 events per 100 patient-years. At last follow-up, 6 (1.5%) patients had died and 10 (2.5%) had undergone heart transplantation. Of these alternative outcomes, 0 deaths and 1 transplant occurred without prior VA event; competing-risk sensitivity analysis was performed and did not impact results.
Figure 1:
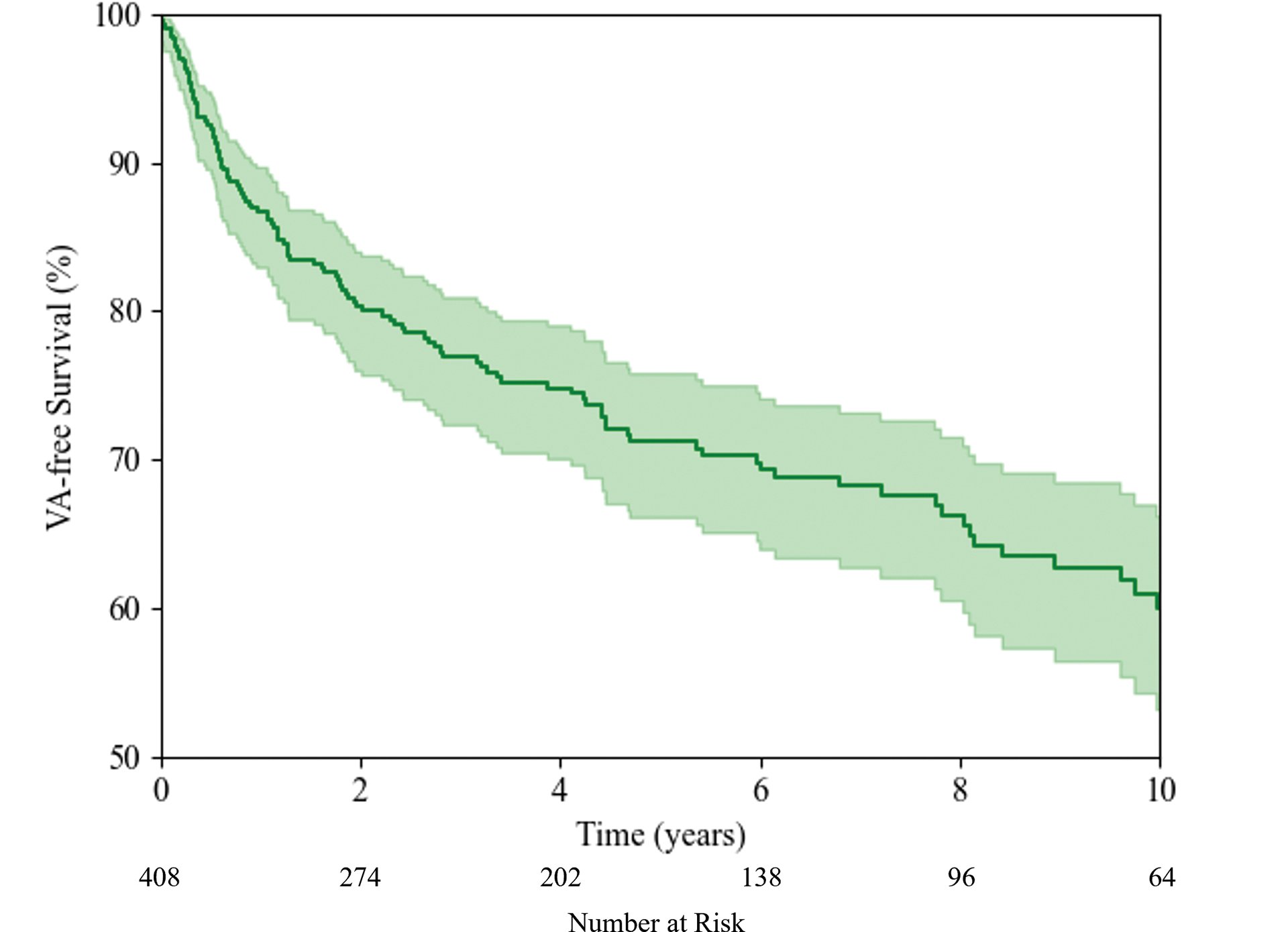
Kaplan-Meier estimate of VA free survival for ARVC patients without prior sustained VA.
Longitudinal Predictive Variables
Table 2 details the number and timing of VA risk factor re-evaluations during clinical follow-up, the distributions of which are presented as histograms in Supplemental Figure I and Supplemental Figure II. Average changes in risk factor values between time of ARVC diagnosis and 5-year follow-up are presented in Table 3. On repeat ambulatory cardiac monitoring assessment, the prevalence of NSVT decreased by 14% and the burden of PVCs decreased by an average of 1,200 PVC per 24 hours. There was a nonsignificant trend towards increased prevalence of moderate to severe RV dysfunction. Sensitivity analysis was performed looking at changes between individual RV functional categories (e.g. normal, mild, moderate, and severe dysfunction) and likewise did not reveal significant changes. There was a small but statistically significant 2% decrease in LVEF. There were no significant changes in the number of TWI on repeat ECG. There was a significant increase of 16% in the prescription rates of anti-arrhythmic medications, but no change in the rates of beta blocker prescriptions. On average, patients decreased their exercise by 4 MET*hr/week. Figure 2 shows the longitudinal trends of the changes in these variables.
Table 2:
Number and timing of VA risk predictor reevaluations during clinical follow-up.
| Evaluation | Number of Pts. w/Additional Evals. | Number of additional Eval. (per patient) | Time of evaluation [IQR] |
|---|---|---|---|
| Ambulatory cardiac monitor (PVC, NSVT) | 294 | 951 (2.3) | 3.0 [1.0, 6.8] |
| ECG (TWI) | 344 | 1,429 (3.5) | 3.5 [1.1, 7.6] |
| Echo (RV function) | 173 | 483 (1.2) | 5.1 [2.1, 9.6] |
| Echo (LVEF) | 251 | 735 (1.8) | 4.4 [1.7, 8.9] |
| Medication review | 104 | 220 (0.5) | 3.6 [0.6, 8.5] |
| Exercise histories | 102 | 102 (0.25) | 4.7 [1.9, 9.0] |
Table 3:
Changes in risk factor values at the time of ARVC diagnosis and at repeat evaluation closest to 5-year follow-up time. Of note, these changes include only patients for whom clinical predictor values were available at both time of diagnosis and at least 3-years of follow-up; the total number of patients is shown.
| Change from Diagnosis to >5 years follow-up | p-value | |
|---|---|---|
| ARVC risk calculator variables | ||
| Log(24 hour PVC count) (n=112) | −0.64±2.5 | 0.009 |
| Presence of NSVT (n=122) | −14% | 0.006 |
| Number of TWI (n=161) | 0.0 [−1.0, 1.0] | 0.456 |
| Presence of RV dysfunction (mod/sev) (n=102) | +6% | 0.181 |
| Other Risk predictors | ||
| LVEF (%) (n=150) | −2.2±7.5 | <0.001 |
| Anti-arrhythmic medication prescribed (n=49) | +16% | 0.044 |
| Beta blocker prescribed (n=49) | +10% | 0.255 |
| Exercise (MET*hr/week) (n=46) | −4 [−42, 7] | 0.016 |
Figure 2:
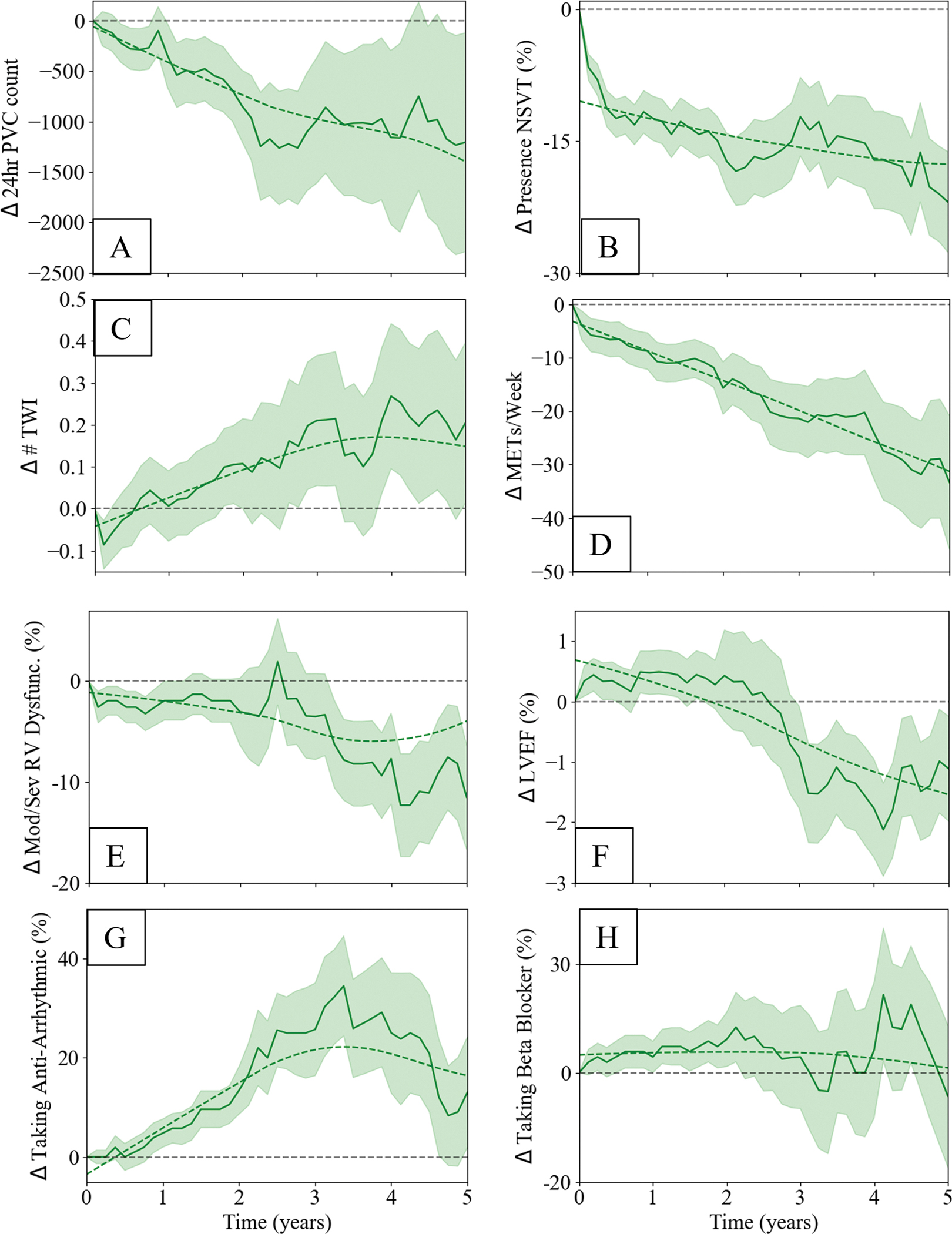
Longitudinal trends in predictors of VA events, presented as the change in predictor value at follow-up relative to time of ARVC diagnosis: A) log of 24-hour PVC burden, B) presence of NSVT on cardiac ambulatory monitoring, C) number of TWI in precordial and inferior leads, D) the extent of strenuous exercise per week, E) presence of moderate or severe RV dysfunction from echocardiography, F) LVEF from echocardiography, G) anti-arrhythmic prescription, and H) beta blocker prescriptions
Longitudinal Risk Prediction
Associations between individual elements of the modified ARVC risk calculator and VA events are presented in Table 4. The C-statistic of the modified ARVC risk calculator for 5-year VA events was 0.76±0.02 and was similar to that of the original ARVC risk calculator (C-statistic 0.78). Figure 3a presents longitudinal trends in model discrimination of 5-year VA events for the 3 risk prediction methods (baseline ARVC risk calculator, updated ARVC risk calculator, and time-varying Cox regression). As shown in Figure 3a, the ability to discriminate VA event risk decreased for the baseline ARVC risk calculator after approximately 3-years and the C-statistic decreased from 0.83±0.03 at time of diagnosis to 0.69±0.06 at 5-years, while the updated ARVC risk calculator and time-varying Cox regression risk remained relatively stable out to 5-years (C-statistics of 0.83±0.03 to 0.79±0.06 and 0.84±0.03 to 0.78±0.06, respectively).
Table 4:
Associations between clinical risk factors included in the modified ARVC risk calculator and 5-year VA event risk. Hazard ratios are presented with 95% confidence intervals.
| Cox proportional hazards regression using baseline variables Hazard ratios [95% CI] |
Time varying Cox regression Hazard ratios [95% CI] |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Univariable | p-value | Multivariable | p-value | Univariable | p-value | Multivariable | p-value |
| Age (years) (per year) | 0.983 [0.972; 0.995] | 0.004 | 0.978 [0.966; 0.989] | <0.001 | 0.983 [0.972; 0.995] | 0.004 | 0.983 [0.971; 0.994] | 0.003 |
| Male sex (vs. female) | 1.843 [1.307; 2.600] | <0.001 | 1.746 [1.234; 2.471] | 0.002 | 1.843 [1.307; 2.600] | <0.001 | 1.730 [1.222; 2.449] | 0.002 |
| Presence of NSVT (vs. absence) | 3.653 [2.434; 5.484] | <0.001 | 2.126 [1.350; 3.347] | 0.001 | 3.012 [2.082; 4.356] | <0.001 | 1.758 [1.165; 2.652] | 0.007 |
| History of cardiac syncope (vs. absence) | 2.197 [1.504; 3.209] | <0.001 | 1.554 [1.050; 2.298] | 0.027 | 2.470 [1.713; 3.562] | <0.001 | 1.794 [1.232; 2.612] | 0.002 |
| # TWI (per lead) | 1.199 [1.107; 1.297] | <0.001 | 1.100 [1.004; 1.206] | 0.04 | 1.176 [1.090; 1.269] | <0.001 | 1.079 [0.994; 1.171] | 0.071 |
| log(24 hour PVC count) | 1.536 [1.366; 1.729] | <0.001 | 1.321 [1.156; 1.510] | <0.001 | 1.381 [1.243; 1.533] | <0.001 | 1.207 [1.080; 1.348] | 0.001 |
| Presence of mod./sev. RV dysfunction (vs absence) | 2.745 [1.922; 3.920] | <0.001 | 1.421 [0.968; 2.084] | 0.073 | 2.961 [2.076; 4.225] | <0.001 | 1.807 [1.239; 2.637] | 0.002 |
Figure 3:
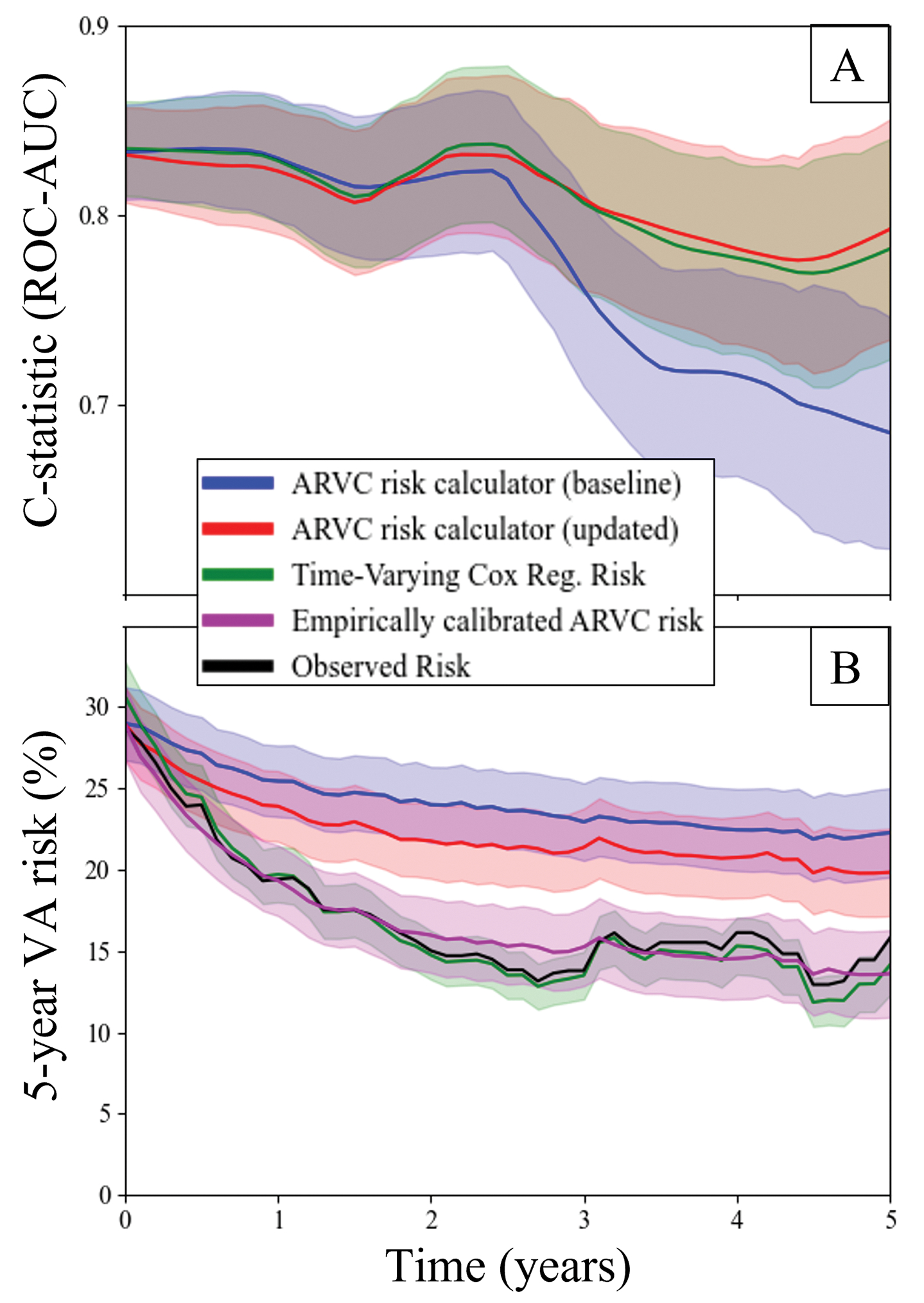
A) Longitudinal changes in model discrimination for (blue) the baseline ARVC risk calculator, (red) the updated ARVC risk calculator, and (green) time-varying Cox regression risk. Plots are shown with LOWESS smoothing and standard errors of the mean. B) Longitudinal calibration between predictions made by (blue) baseline ARVC risk calculator, (red) updated ARVC risk calculator, (green) time-varying cox regression risk, compared to observed risk (black). The updated ARVC risk calculator risk was recalibrated using an empiric exponential decay function (magenta). Here observed risk is shown with 95% confidence intervals, and model predictions are shown with standard errors of the mean.
Mean VA risk predictions from the three models are shown in Figure 3b, where they are compared to observed VA risk. Observed 5-year risk decreased from 29% to 16% between the time of initial ARVC diagnosis and at 5-year follow-up. While all models showed a decrease in predicted 5-year VA risk at 5-year follow-up, these decreases were smaller in magnitude for both baseline ARVC risk calculator (29% decreasing to 22%) and updated ARVC risk calculator (29% decreasing to 20%). The time-varying Cox regression risk predictions (31% decreasing to 14%) more closely matched the observed drop in 5-year risk. Risk predictions from the updated ARVC risk calculator were recalibrated using an empiric exponential decay function, resulting in close approximation of observed risk (29% decreasing to 14%). The average risk discrepancy estimated using this calibration model was +6%. Risk discrepancies in the low, intermediate, and high-risk groups were +2%, +9%, and +13%, respectively. Subgroup calibration plots are shown in Supplemental Figure III, and details of empiric calibration models are presented in Supplemental Table III.
Discussion
In this study we leveraged a large, deeply phenotyped, multicenter cohort of ARVC patients with long-term follow-up to characterize how VA risk factors change over time and to define how these changes can be incorporated into models for longitudinal VA risk prediction. Our findings shed important new insights into the dynamic nature of the disease course of ARVC following initial diagnosis. In particular, we demonstrated the importance of changes in ventricular ectopy, with both prevalence of NSVT and PVC burden acting as independent risk factors for VA events that decrease during follow-up. Overall likelihood of primary VA events likewise decreased over time. Applying the same baseline ARVC risk calculator prediction to follow-up evaluations resulted in decreasing VA risk discrimination after 3 years. However, this decrement was negated by updating the ARVC risk calculator prediction with changes in risk factor values (i.e. assessing 5-year VA risk using the ARVC risk calculator and the most recent set of available risk factor data). Mean risk for initial VA event during the subsequent 5-year period was overestimated by an average of +6% compared to both observed risk, though this overestimate was smaller in low-risk patients. We created a time-varying Cox regression model for predicting 5-year VA risk that maintained excellent discrimination and accuracy at 5-year follow-up.
Comparison to other study findings
While there have been a handful of studies reporting longitudinal changes in individual ARVC risk factors, our study represents the first examination of how these risk factors change in concert with one another. Similar to our findings, one recent observational study examining patients with multiple Holter monitors found that the average burden of PVC decreased after initial diagnosis10. This study likewise demonstrated the importance of changes in PVC count, with both presence of NSVT and increase in PVC burden independently identifying increased risk for VA events in the year following assessment. Cappelletto et al. likewise found that both NSVT and PVC burden decreased progressively at both 2-year and 8-year follow-up in their cohort of patients with repeat Holter monitoring 17, and that NSVT remained an important independent risk factor for VA at follow-up. It is unclear whether these changes are part of the natural disease course in ARVC, or if decreased ventricular ectopy is the result of initiating pharmacologic therapy and lifestyle modification. It is also plausible that the observed improvements in electrophysiologic properties may be exaggerated due to selection bias, as both PVC count and NSVT are important arrhythmic components of the ARVC diagnostic criteria.
For patients with repeat echocardiographic assessment, we found that cardiac function was stable between ARVC diagnosis and 5-year follow-up. On average, patients did not have progressive RV dysfunction during that period. While patients did demonstrate a statistically significant 2% decrease in LVEF, this small change is unlikely to be clinically significant. These findings are consistent with other studies that have looked at changes in cardiac function in ARVC over time. In a smaller study of ARVC patients with serial echocardiograms, Malik et al. found small but significant decreases in LVEF without significant changes in RV fractional area change over a similar time frame18. Contrasting this, Taha et al. found that RV fractional area change decreased by 5% over 7-year follow-up of ARVC patients with serial imaging19. Kalantarian et al. found that about a quarter of ARVC patients had a drop of at least 10% in RV fractional area change over 10-year follow-up 11. Thus, significant functional cardiac changes in ARVC seem to occur over longer time scales (> 5 years) than our present study was able to examine. It is also possible that our evaluation of RV function as a dichotomous rather than continuous variable (e.g. fractional area change or ejection fraction) may have overlooked more subtle, early progression of RV dysfunction. We likewise did not explore more sensitive markers such as echocardiographic or CMR based RV strain that have been shown to be associated with progression of RV dysfunction18. However, we did not find that substitution of RVEF with a categorical definition of RV dysfunction negatively impacted the ARVC risk calculator’s ability to discriminate VA risk, suggesting that these early changes are less important for predicting incident VA. This is consistent with prior studies showing that RV strain did not add incremental value to prediction of VA over broader assessments of RV dysfunction20.
We also found that the number of TWI on ECG was relatively stable out to 5-years of follow-up. These findings are consistent with prior work demonstrating that while TWI in both the inferior and precordial leads are common, they change little by around 5-years of follow-up 21, 22. In contrast, studies examining longer-term follow-up with serial ECGs out to 10-years have demonstrated increased numbers of ECG leads with TWI 11, 23, 24. As with cardiac functional changes, these findings suggest that the progression of ECG changes, and thus the electrophysiologic and structural changes they reflect, likely change over longer time spans (>5 years) than the present study was able to examine.
Longitudinal trends in VA risk and risk prediction
We found that average risk for first sustained VA event decreased by nearly half (absolute risk reduction of 13%) between initial evaluation and 5-year clinical follow-up (Figure 3, black line). This may in part be due to the selection bias inherent to this type of analysis. Those high-risk patients present in the initial cohort who go on to have VA events are by definition no longer at risk for a first VA event. They are thus removed from the pool of patients for whom risk of initial VA events are subsequently assessed. This is reflected by the negative trend in risk predicted by the baseline ARVC risk calculator (Figure 3, blue lines) and accounts for an approximately 7% decrease in average risk by 5-years. This only partially explains the total 13% decrease in observed risk, however. The discrimination of the baseline ARVC risk calculator also drops off significantly after 3-years, suggesting that there is also heterogeneity in the way that individual patient risk changes over time. Accounting for changing patient characteristics by recalculating the predicted risk with the most recent set of risk factor data results in significantly improved discrimination of VA likelihood (Figure 3, red lines) but a persistent overestimation of mean risk (+6% at 5-year follow-up). In contrast, the time-varying Cox-regression model for risk prediction had both good discrimination and well calibrated mean risk (Figure 3, green lines). This model takes advantage of complete knowledge of the baseline hazard function (e.g. the instantaneous VA risk at all follow-up times for a patient with null risk factors), and thus incorporates empiric changes to risk that exceed those accounted for by the included VA risk factors. Similarly, we were able to recalibrate the updated ARVC risk calculator predictions using an empiric exponential decay function (Figure 3, magenta lines), which resulted in both excellent discrimination and closely calibrated mean risk. Thus, there appear to be three distinct sources of decreasing VA risk: survivorship bias, improving risk factors included within the ARVC risk calculator (age, NSVT, and PVC count), and additional risk modifiers that are currently unaccounted for by the ARVC risk calculator but that do not impact risk discrimination.
Two risk modifiers that may decrease longitudinal VA risk but are not included in the ARVC risk calculator are reductions in exercise and initiation of medical therapy. We found that patients significantly reduced the amount and intensity of their exercise between initial diagnosis and 5-year clinical follow-up (Figure 2, panel D). Prior studies have shown that competitive sports activity is associated with as much as a 5-fold increase in risk for SCD in young adults 25 26, and that this association is dose-dependent 27, 28. Even recreational sports contribute significantly to risk of VA and SCD 29. In this context, our results support decreasing exercise as a plausible mechanism for reducing risk for VA. We also found that patients in our cohort were more likely to be prescribed anti-arrhythmic medications at 5-year follow-up compared to at the time of initial diagnosis (Figure 2, panel G). While evidence for the use of anti-arrhythmic medications in ARVC is mixed 30, observational data suggests that these medications, particularly amiodarone and sotalol, may reduce the rate of VA events in patients with high burdens of PVC and NSVT 31–34. We did not find that rates of beta-blocker prescriptions changed significantly between initial ARVC diagnosis and 5-year follow-up. This may be because of the moderately high rates (~40%) of baseline beta blocker prescriptions, and the lack of strong evidence supporting their efficacy in isolated right sided dysfunction or for prevention of VA events26. In addition, it has been hypothesized that episodes of acute inflammation elicited by environmental triggers may play a role in modulating disease progression35. As inflammation increases both VA risk and symptom burden, it follows that ARVC diagnosis is most likely to be made during an inflammatory episode, thus leading to the observed pattern of heightened initial VA risk followed by risk attenuation as the episode recedes.
Clinical Implications
Our time-varying Cox regression model provided a combination of strong discrimination and accurate VA risk prediction. However, its clinical use would likely be cumbersome due to the need for providers to enter a significant quantity of risk factor data in order to generate risk predictions. Ultimately this could be achieved via integration into electronic health records systems. Alternatively, our findings suggest that the ARVC risk calculator remains a useful clinical tool for discriminating between low- and high-risk patients during follow-up evaluation, provided that predictions are made using updated risk factor data. Predictions made by the ARVC risk calculator overestimate the observed risk at follow-up evaluations, the average magnitude of which was +6%. This overestimation is smaller (+2%) in patients with low baseline risk and larger in patients with high baseline risk (+13%) (Supplemental Table III). Since those patients at low baseline risk are least likely to have ICD placement at time of ARVC diagnosis, the updated ARVC risk calculator therefore performed best in the population for whom longitudinal VA risk reassessment was most relevant.
Additionally, we present a modified version of the ARVC risk calculator which makes use of a dichotomous RV dysfunction variable, rather than continuous RVEF. This modification did not decrease the model’s discrimination in this cohort and has the added benefit of eliminating the score’s reliance on CMR imaging data which may be unavailable at follow-up (particularly after ICD implantation) or granular RV fractional area change which may not be routinely available in clinical echocardiograms. External validation will also be required before this modified risk prediction tool should be used clinically.
Finally, our findings are consistent with the hypothesis that reduction in exercise and initiation of anti-arrhythmic medications may help to reduce the likelihood of VA events. While Bosman et al examined the incremental value of adding exercise to the ARVC risk calculator and found no improvement in VA risk prediction, their analyses were restricted to risk prediction at the time of initial ARVC diagnosis28. It is possible that reducing exercise and initiating anti-arrhythmic medications may be important for improving individualized, longitudinal risk predictions. That said, in one small cohort of athletic ARVC patients, ARVC risk calculator predictions also seemed to hold despite clinical detraining 36. Further analyses of cohorts with more complete exercise history and medication review data are therefore needed to clarify the incremental value of these variables in longitudinal VA risk prediction. Regardless, the updated ARVC risk calculator had excellent discrimination without inclusion of either exercise or medication data.
Limitations
We acknowledge the observational nature of this study as a limitation. All longitudinal reassessments of risk predictors were obtained at the discretion of the local clinicians introducing possible observation bias. However, this observation bias most likely takes the form of increased surveillance in high-risk patients and those with clinical symptoms, which represent the population for whom VA risk prediction is of most relevance. Additionally, while many repeat diagnostic tests were available during follow-up, the number of patients for whom complete exercise histories and longitudinal medication reviews were available represent a small fraction of the overall cohort, and may have therefore increased the risk of type 2 error (e.g. our failure to detect change in beta blocker prescription rate) and/or be less representative of the full cohort. To confirm our hypotheses that the differences between observed VA event rates and uncalibrated ARVC risk predictions are due to these risk modifiers, further studies with more complete exercise and medication review data should be performed. Finally, our study population was drawn from tertiary, academic centers from North America and Northern Europe which may have created a referral bias that could lead to overestimation of VA risk in a community-derived population. External validation of our model for longitudinal VA risk assessment is essential to confirm its clinical utility. Additionally, as in the original ARVC risk calculator, we used a surrogate composite endpoint that included appropriate ICD therapy to infer risk of SCD. While clinically recognized as significant arrhythmic events, ICD therapies are an imperfect substitute for SCD37. As a further limitation of our multi-center, longitudinal registry-based study of a rare disease, we do not have granular data regarding the breakdown of these ICD therapies into anti-tachycardic pacing versus appropriate shock, or of the programmed detection times for therapies.
Conclusions
In the present study, we leveraged a well-characterized, international multi-center cohort of ARVC patients with long-term clinical follow-up to explore the ways in which risk factors for VA change over time, how these changing risk factors impact overall rates of sustained VA, and how well current risk assessment tools perform on serial evaluation. On average, we found that ventricular ectopy including both burden of PVCs and prevalence of NSVT decreased significantly between time of diagnosis and 5-year follow-up, while structural and functional risk factors including RV function and number of TWI on ECG remained largely static. We found that updating the ARVC risk prediction using the most recent set of VA risk factors was important in maintaining discrimination during follow-up. Additionally, observed 5-year VA risk decreased quickly relative to predicted risk, suggesting the influence of risk modifiers that are not explicitly included in the ARVC risk calculator. Mean VA risk was overestimated by +6% at 5-year follow-up, and this overestimation should be accounted for when providing clinical risk assessments.
Supplementary Material
What is known?
ARVC is a genetic cardiomyopathy associated with a high burden of ventricular arrhythmias.
The ARVC risk calculator is a clinical tool used for identifying high-risk patients who may benefit from a primary prevention ICD.
What the study adds?
Following initial ARVC diagnosis, average risk for developing VA events decreases with time, as does the burden of PVCs and prevalence of NSVT.
While the ARVC risk calculator maintains excellent VA risk discrimination out to 5 years of follow-up, it should be recalculated at each follow-up using the most recent set of clinical risk factors.
VA risk predictions from the ARVC risk calculator are overestimated by an average of +6% by 5 years of follow-up.
Sources of Funding:
This research was funded by multiple generous sources, including the Johns Hopkins Cardiology NIH T32HL007227 training grant (RTC), the Leonie-Wild Foundation, the Wilton W. Webster Fellowship from the Heart Rhythm Society (AG), the Netherlands Heart Foundation grant 2015T058 and UMC Utrecht Fellowship Clinical Research Talent, and the Young Talent Program CVON2012-10 PREDICT (ASJMtR) and PREDICT2 (AAMW and JPvT). We acknowledge the support from the Netherlands Cardiovascular Research Initiative, an initiative with support of the Netherlands Heart Foundation, grant nos.: CVON2012-10 PREDICT, CVON2015-12 eDETECT. The Netherlands ACM Registry is supported by the Netherlands Heart Institute (project 06901). The Johns Hopkins ARVC Program is supported by the Leyla Erkan Family Fund for ARVD Research, the Dr. Francis P. Chiramonte Private Foundation, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Nonstandard Abbreviations and Acronyms
- ARVC
Arrhythmogenic right ventricular cardiomyopathy
- VA
Ventricular arrhythmias
- SCD
Sudden cardiac death
- ICD
Implantable cardioverter defibrillator
- RVEF
Right ventricular ejection fraction
- TWI
T-wave inversions
- ECG
Electrocardiogram
- NSVT
Non-sustained ventricular tachycardia
- PVC
Premature ventricular contraction
- LVEF
Left ventricular ejection fraction
- VT
Ventricular tachycardia
- ROC-AUC
Receiver-operator curve area under the curve
- IQR
Interquartile range
- CMR
Cardiac magnetic resonance
Footnotes
Disclosures: Dr. Calkins consults for Medtronic Inc., Biosense Webster, Pfizer, StrideBio, and Abbott. Ms. Murray consults for MyGeneCounsel. Dr. James consults for Pfizer, Inc, Tenaya Inc., and StrideBio, Inc. Dr. Calkins, Dr. James, and Ms. Tichnell receive research support from Boston Scientific Corp. Dr. Tandri receives research support from Abbott. Dr. Yap consults for Boston Scientific Corp. Dr Wilde consults for LQTherapeutics and ARMGO.
Supplemental Materials:
Reference 38
References:
- 1.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rampazzo A, Nava A, Danieli GA, Buja G, Daliento L, Fasoli G, Scognamiglio R, Corrado D, Thiene G. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet. 1994;3:959–962. [DOI] [PubMed] [Google Scholar]
- 3.Peters S, Trümmel M, Meyners W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int J Cardiol. 2004;97:499–501. [DOI] [PubMed] [Google Scholar]
- 4.Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, Mellor G, Merghani A, Malhotra A, Behr E, et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J Am Coll Cardiol. 2016;67:2108–2115. [DOI] [PubMed] [Google Scholar]
- 5.Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, Bhonsale A, Kamel IR, Zimmerman SL, Judge DP, Crosson J, et al. Implantable Cardioverter-Defibrillator Therapy in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Predictors of Appropriate Therapy, Outcomes, and Complications. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, de Groot JR. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. [DOI] [PubMed] [Google Scholar]
- 7.Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie ØH, Saguner AM, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, Barison A, Romani S, Lesizza P, Fabris E, et al. Comparison of different prediction models for the indication of implanted cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy. ESC Heart Fail. 2020;7:4080–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, Loh P, Russell SD, Velthuis BK, Judge DP, et al. Evaluation of Structural Progression in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. JAMA Cardiol. 2017;2:293–302. [DOI] [PubMed] [Google Scholar]
- 10.Gasperetti A, Cappelletto C, Carrick R, Targetti M, Tichnell C, Martino A, Murray B, Compagnucci P, Stolfo D, Bisson J, et al. Association of Premature Ventricular Contraction Burden on Serial Holter monitoring with Arrhythmic Risk in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy. JAMA Cardiology. 2022;7(4):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantarian S, Åström Aneq M, Svetlichnaya J, Sharma S, Vittinghoff E, Klein L, Scheinman MM. Long-Term Electrocardiographic and Echocardiographic Progression of Arrhythmogenic Right Ventricular Cardiomyopathy and Their Correlation With Ventricular Tachyarrhythmias. Circ Heart Fail. 2021;14:e008121. [DOI] [PubMed] [Google Scholar]
- 12.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. [DOI] [PubMed] [Google Scholar]
- 13.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 16.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 17.Cappelletto C, Stolfo D, De Luca A, Pinamonti B, Barbati G, Pivetta A, Gobbo M, Brun F, Merlo M, Sinagra G. Lifelong arrhythmic risk stratification in arrhythmogenic right ventricular cardiomyopathy: distribution of events and impact of periodical reassessment. Europace. 2018;20:f20–f29. [DOI] [PubMed] [Google Scholar]
- 18.Malik N, Win S, James CA, Kutty S, Mukherjee M, Gilotra NA, Tichnell C, Murray B, Agafonova J, Tandri H, et al. Right Ventricular Strain Predicts Structural Disease Progression in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy. J Am Heart Assoc. 2020;9:e015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taha K, Mast TP, Cramer MJ, van der Heijden JF, Asselbergs FW, Doevendans PA, Teske AJ. Evaluation of Disease Progression in Arrhythmogenic Cardiomyopathy: The Change of Echocardiographic Deformation Characteristics Over Time. JACC Cardiovasc Imaging. 2020;13:631–634. [DOI] [PubMed] [Google Scholar]
- 20.Bourfiss M, Prakken NHJ, James CA, Planken RN, Boekholdt SM, Ahmetagic D, van den Berg MP, Tichnell C, Van der Heijden JF, Loh P, et al. Prognostic value of strain by feature-tracking cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022;jeac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saguner AM, Ganahl S, Kraus A, Baldinger SH, Akdis D, Saguner AR, Wolber T, Haegeli LM, Steffel J, Krasniqi N, et al. Electrocardiographic features of disease progression in arrhythmogenic right ventricular cardiomyopathy/dysplasia. BMC Cardiovasc Disord. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccini JP, Nasir K, Bomma C, Tandri H, Dalal D, Tichnell C, James C, Crosson J, Calkins H. Electrocardiographic findings over time in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2005;96:122–126. [DOI] [PubMed] [Google Scholar]
- 23.Gallo C, Blandino A, Giustetto C, Anselmino M, Castagno D, Richiardi E, Gaita F. Arrhythmogenic right ventricular cardiomyopathy: ECG progression over time and correlation with long-term follow-up. J Cardiovasc Med (Hagerstown). 2016;17:418–424. [DOI] [PubMed] [Google Scholar]
- 24.Jaoude SA, Leclercq JF, Coumel P. Progressive ECG changes in arrhythmogenic right ventricular disease. Evidence for an evolving disease. Eur Heart J. 1996;17:1717–1722. [DOI] [PubMed] [Google Scholar]
- 25.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. [DOI] [PubMed] [Google Scholar]
- 26.Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. [DOI] [PubMed] [Google Scholar]
- 27.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosman LP, Wang W, Lie ØH, van Lint FHM, Rootwelt-Norberg C, Murray B, Tichnell C, Cadrin-Tourigny J, van Tintelen JP, et al. Integrating Exercise Into Personalized Ventricular Arrhythmia Risk Prediction in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ Arrhythm Electrophysiol. 2022;15:e010221. [DOI] [PubMed] [Google Scholar]
- 29.Ruwald AC, Marcus F, Estes NA 3rd, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. [DOI] [PubMed] [Google Scholar]
- 31.Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation. 1992;86:29–37. [DOI] [PubMed] [Google Scholar]
- 32.Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, Estes NA 3rd, Marcus F, Scheinman MM. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. 2009;54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ermakov S, Gerstenfeld EP, Svetlichnaya Y, Scheinman MM. Use of flecainide in combination antiarrhythmic therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2017;14:564–569. [DOI] [PubMed] [Google Scholar]
- 34.Rolland T, Badenco N, Maupain C, Duthoit G, Waintraub X, Laredo M, Himbert C, Frank R, Hidden-Lucet F, Gandjbakhch E. Safety and efficacy of flecainide associated with beta-blockers in arrhythmogenic right ventricular cardiomyopathy. Europace. 2021;4(2):278–284. [DOI] [PubMed] [Google Scholar]
- 35.Asatryan B, Asimaki A, Landstrom AP, Khanji MY, Odening KE, Cooper LT, Marchlinski FE, Gelzer AR, Semsarian C, Reichlin T, et al. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation. 2021;144:1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasperetti A, Dello Russo A, Busana M, Dessanai M, Pizzamiglio F, Saguner AM, Te Riele A, Sommariva E, Vettor G, Bosman L, et al. Novel risk calculator performance in athletes with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17:1251–1259. [DOI] [PubMed] [Google Scholar]
- 37.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 38.Collett D Modelling Survival Data in Medical Research. 3rd ed. Boca Raton, FL: CRC Press: Taylor & Francis Group; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


