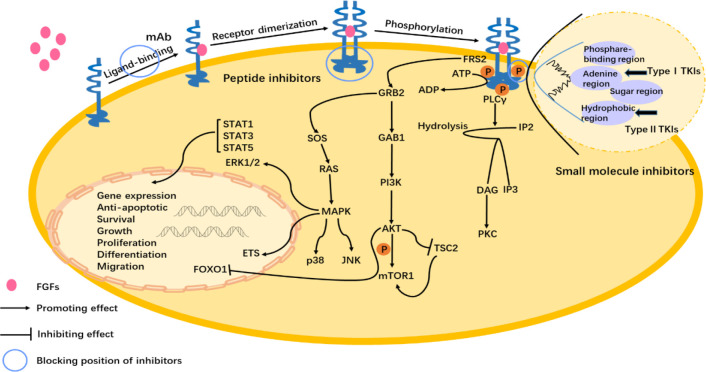
Figure 3.
FGFs/FGFR1 signal pathway and the mechanisms of FGFR1 inhibitors. FGFs binding to the FGFR1 induces dimerization and the subsequent phosphorylation of the intracellular tyrosine kinase domain. Activation of downstream signaling occurs via FGFR substrate 2α (FRS2α), which is constitutively associated with the juxtamembrane region of FGFR. Phosphorylated FRS2 recruits the adaptor protein, growth factor receptor-bound protein 2 (GRB2), which then recruits the guanine nucleotide exchange factor SOS. The recruited SOS activates the RAS GTPase, which then activates the MAPK pathway. MAPK activates members of the ETS transcription factor family, ERK1/2, p38, and Jun N-terminal kinase pathways (JNK). The recruited GRB2 as well as recruits the adaptor protein GAB1, which then activates the PI3K, and after that phosphorylates the AKT. Next, then AKT has various activities including activation of the mTOR complex 1 by inhibition of the cytosolic tuberous sclerosis complex 2 (TSC2) and phosphorylation. And AKT pathway inhibits the activity of the forkhead box class transcription factor (FOXO1) bringing about it exiting the nucleus. Phospholipase C (PLC) binds to a phosphotyrosine and hydrolyzes phosphatidylinositol 4,5-bisphosphate (IP2) to phosphatidylinositol 3,4,5-triphosphate (IP3) and diacylglycerol (DAG), which then activates protein kinase C (PKC). The target gene expression is regulated by the activity of the signal transducer and activator of transcription STAT1 (signal transducer and activator of transcription), STAT3, and STAT5. The abnormal activation of the FGFs/FGFR pathway has an influence on physiological activity, such as anti-apoptotic, survival, and growth of cells (1–3). The mechanisms have been shown in the figure of small molecule inhibitors, peptide inhibitors, and monoclonal antibodies.