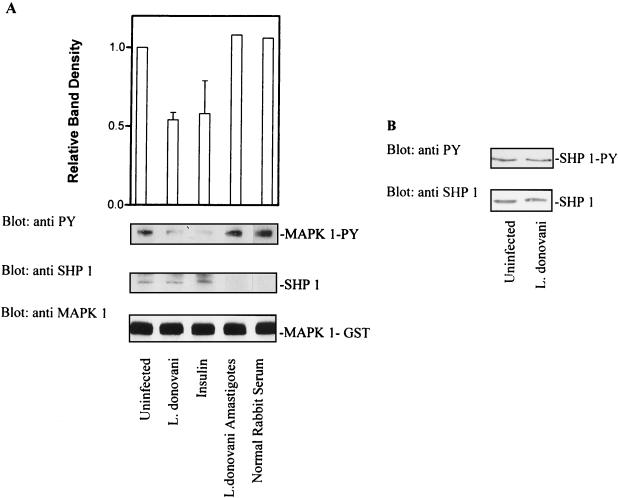
FIG. 8.
L. donovani infection increases the specific activity of SHP-1 without affecting either tyrosine phosphorylation status or cellular levels of enzyme. (A) Cells were infected with leishmania for 17 h. Control and infected cells were lysed in buffer as described in Materials and Methods. Detergent-solubilized proteins were immunoprecipitated with antibody to SHP-1. Immunoprecipitated SHP-1 was incubated with autophosphorylated MAP kinase-1–GST (as described in Materials and Methods) for 1 h at 30°C. Reactions were stopped by adding SDS sample buffer. Phosphatase activity was assessed by immunoblotting with antiphosphotyrosine (anti PY) antibodies. The autoluminogram shown was analyzed by densitometry. The blots were then stripped and reprobed with anti-SHP-1 to determine the level of immunoprecipitated SHP-1. The same membranes were again stripped and reprobed with anti-MAP kinase (anti MAPK 1) to assess the input of MAP kinase-1–GST. (B) (Top) Cells infected with leishmania (17 h) were lysed in buffer, immunoprecipitated with anti-SHP-1, and immunoblotted with antiphosphotyrosine antibodies to assess the tyrosine phosphorylation status of SHP-1. (Bottom) Cells infected with leishmania (17 h) were lysed in modified RIPA buffer, and solubilized proteins were separated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) followed by immunoblotting. Total cellular levels of SHP-1 were measured by immunoblotting with anti-SHP-1 antibody. The data shown in lanes with error bars are the mean and standard deviation of results obtained in three independent experiments. Other results are control results from one experiment.