Abstract

In recent decades, organ-on-chip devices have gained substantial interest as an alternative for studying the pathophysiological processes relevant to drug screening. Micropumps are being utilized to simulate the in vivo physiological fluid flow more realistically in these organ-on-chip devices. Micropumps play a crucial role in pumping, perfusion, and circulation of fluids in various microdevices such as on-chip PCR, DNA microarrays, miniature bioreactor cell separation, and lab-on-chip biosensing platforms. With the rapid growth in technology, efficient pumping for proper circulation of media and nutrients has become imperative. In this study, we have described the design and development of an open-source impedance micropump for continuous perfusion of nutrient medium in a liver-on-chip prototype. This micropump is controlled via an integrated microcontroller board, with an observed flow rate ranging from 0.2 to 2 mL/min. Google Sketchup 2020 and DLP 3D printing were used to fabricate small precise parts of the impedance micropump. The flow rate was measured to characterize the actuating performance of the micropump. The poly-dimethyl siloxane-based liver-on-chip prototype has been fabricated using a soft photolithography procedure. Further, a study of continuous perfusion of culture medium through the liver-on-chip containing the Hepg2 cell line was successfully performed by integrating it with the impedance micropump. Hoechst staining and Alamar Blue observed cell viability to confirm the healthy cell growth inside the liver-on-chip microfluidic chip. The compactness of the overall setup allows it to fit in a Petri plate, eliminating chances of contamination while cell handling.
Introduction
The organ-on-a-chip (OOC) device requires a continuous flow of media/nutrients inside the microchannels to maintain dynamic cell culturing conditions. To achieve a precise flow, micropumps have been used for the controlled pumping of fluids in the microfluidic channels. Several types of micropumps have been reported, developed, and applied in research since the late 90s. One of the major advantages of using a micropump is its miniature design and potability as compared to that of the bulky peristaltic or syringe pump. Most conventional micropumps consist of valves, diaphragms, pneumatic valves, or piezoelectric elements as their actuation generating components. This directly affects the overall cost and power consumption of the micropump. Table 1 shows the reported micropumps with different actuation principles and operating mechanisms. Most of the micropumps have valves that ensure the unidirectional flow of fluids and need to be synchronized with the actuation mechanism to initiate a flow. Additionally, these valve structures need to be precisely fabricated, which mostly requires microfabrication facilities that directly affect the overall cost of the device. Some micropumps, such as thermo-pneumatic ones, operate on 10–15 V, with high current consumption to run microheaters for their operation.1 On the other hand, a piezoelectric-based micropump requires precisely cut piezoelectric materials and DC to AC conversion for its actuation.2 A peristaltic micropump is a valveless micropump that requires similar electrical or mechanical components to the abovementioned micropumps and sometimes requires spring-loaded rollers for generating the peristaltic wave, which in turn requires a geared DC motor or a heavy-duty stepper motor.3
Table 1. Comparative Description of Various Reported Conventional Micropump Systems Based on Their Actuation Principles, Fabrication Methods, and Cost Concerns.
| valves | fabrication method | fabrication cost | power source | |
|---|---|---|---|---|
| thermopneumatic micropump | present | micromachining and MEMS fabrication | moderate | 10–15 V DC (high current rating) |
| piezoelectric micropump | present | micromachining and piezoelectric material | high | 5–10 V (DC–AC conversion) |
| electromagnetic micropump | present | micromachining and MEMS fabrication | moderate | 5–10 V DC |
| peristaltic micropump | absent | micromachining, piezoelectric, electromagnetic, pneumatic, and so forth | high | it depends upon the fabrication level |
| impedance micropump (proposed) | absent | normal fabrication | low | 1–3 V DC |
The micro-impedance class of micropumps projected in this study comes under the valveless pumps category and may be used in the domain of micro- and macro-fluidic systems.4 Impedance pumps are a kind of valveless pump and are generally of sizeable dimensions. While operating on an unleveled surface, the effect of gravity becomes more significant, and hence, the micropump cannot maintain a stable flow rate. The influence of gravity makes it difficult for the pump to generate enough suction to pump a specific fluid volume against gravity. The dimensions of the proposed impedance micropump are kept in a range (1–20 mm) to overcome these issues, thus providing accurate and controlled pumping. This micropump may perform the planned crucial functions of nutrient supply and waste removal in microfluidic devices concerned with cell culturing and tissue engineering. Furthermore, the impedance pump can easily be manufactured using injection molding or 3D printing techniques. Its simple design and lack of complicated components make it ideal for mass manufacturing. The impedance micropump does not require any complex components such as blades, microvalves, piezo elements, or high electric fields, making it safe for studies involving perfusion and handling of sensitive biomolecules and cell-based studies. Accordingly, it is typically straightforward to integrate an optical evanescent wave absorbance or a localized surface plasmon resonance-oriented microfluidic biosensor module to the proposed micropump in a single microfluidic biosensor design.5−9 Adjusting simple parameters such as the excitation frequency or tapping location can change the flow’s direction and provide a versatile flow output. Such characteristics make the impedance micropump well-suited to various applications ranging from point-of-care devices, drug toxicity testing, organ-on-a-chip, and drug delivery with precisely controlled flow rates. During the past few years, there has been an evolution in 3D printing technologies, making them more reliable and precise over time. Stereolithography (SLA) 3D printers serve wide applications, which use near UV lasers and photocurable resins for 3D printing objects and can provide higher printing resolutions of 30–50 μm. One such category of 3D printer uses a UV LCD screen to 3D print objects, known as digital light processing (DLP) 3D printers. The DLP printer is used to 3D print impedance micropump parts 3D designed using CAD software, provides precise structural details without fail, and can print very intricate designs and structures with a resolution of around (1 μm).10 The impedance micropump was 3D printed with triplicate copies with a precise resolution of around ±1 μm. After assembling the triplicate copies of the impedance micropumps, there were no significant variations observed in the structural as well as operational attributes. It resulted in an adequate reproducible device construction outcome, as well as the repeatable pumping characteristics of the micropump. OOC is a 3D in vitro physiological microfluidic system aiming to recreate the in vivo conditions found inside the organ/tissue on a microfluidic chip.11 Similarly, a liver-on-a-chip is a high-throughput system capable of imitating conditions of hepatocytes and the dynamic physicochemical hepatic environment. These devices have the potential to accelerate the existing drug development pipeline by providing an alternative to the animal models.12 Liver-on-a-chip devices have shown promising results in mimicking in vivo conditions by recreating the sinusoidal structure of the liver while maintaining high cell viability and cellular phenotypes and emulating native liver functions.13 Here, we have integrated the impedance micropump to a liver-on-chip device designed to study drug toxicity in liver cells. The purpose of the micropump is to maintain the optimal media flow, which reaches the cells through perfusion.
The intended OOC microfluidics has been an essential explored domain for the past few years and has become a hot topic of current research. The field promises to eradicate animal models for drug testing and drug development, which also does not predict good results in humans. For simulating the microenvironment (existing inside the human body), these OOC microfluidic chips contain seeded human cells, which provide mostly accurate results compared to the animal models.14,15 One research group performed drug toxicity testing over the 3D cell culture platform and utilized the conventional peristaltic pump for perfusing culture media through the platform. They fabricated the microfluidic device using polymethyl methacrylate and poly-dimethyl siloxane (PDMS) after seeding the HT29 cell line and cultured 3D spheroids within the microfluidic channels for drug toxicity testing.16 In another approach, a research group designed and tested a novel microfluidic device for oxygenating blood samples using a custom-made setup, where they used a syringe pump as a pumping device to pump the swine venous blood inside the designed microfluidic device.17 Considering the crucial role of pumps in the microfluidic domain, we identified a need to develop a cost-effective alternative that consumes low power, are small, and have low operational cost. The proposed low-cost, miniature impedance micropump addresses these crucial needs in this regard. It can be effectively planned as a miniature heart connected to the cell-seeded microfluidic chip for perfusion and circulation of the culture media.
Theoretical Principle
The actuation in the impedance micropump occurs by linear beating of the minuscule tapper head and is attained by reciprocating machinery. This mechanism is known as the also Scotch and Yoke mechanism. For the production of diminutive reciprocating machinery, the minute motor shaft should possess eccentricity according to the preferred linear displacement. For the impedance micropump mentioned in this study, a linear displacement of 0.5 mm is needed, for which the shaft of the minuscule motor is introduced with a 0.5 mm bent in such a way that the shaft of the motor rotates eccentrically. The flow inside the impedance micropump depends upon the viscosity of the sample fluid, which can be determined by relating the elastic boundary shear stress with the mean (axial) flow along with continuity and momentum equations as18
| 1 |
| 2 |
Here, x is the length of the elastic tube, t is the time, A is the flexible tube cross-sectional area, u is the mean flow velocity at cross-section A, P is the transmural pressure, and ρ is the density. F is the quasi-viscous source in the momentum equation that determines the friction between the fluid and the elastic tube wall. The physical model of the impedance pump developed in the present study utilizes a two-dimensional model19 with the Navier–Stokes equations, which are written as
| 3 |
| 4 |
In the equations above, v is the flow velocity of the sample fluid in the x–y plane and p is the pressure.
Design and Fabrication
3D Design, Fabrication, and Assembly of the Impedance Micropump
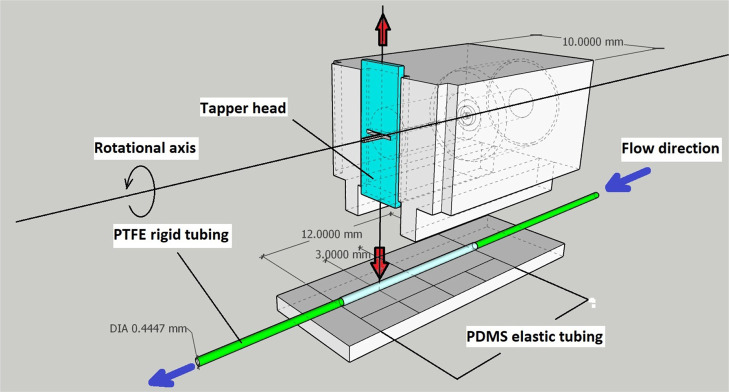
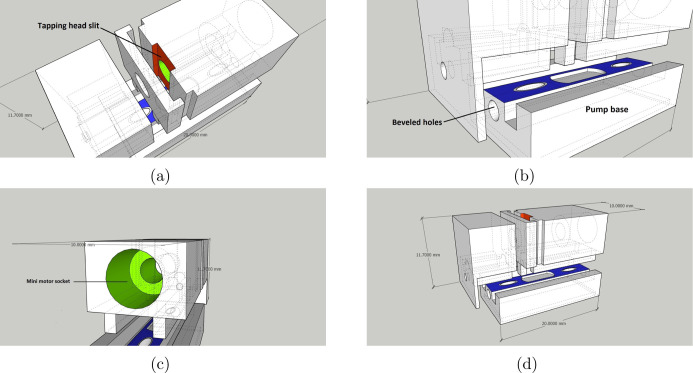
While designing the impedance micropump, we prioritized simple assembly and easy handling. Figure 1 describes the 3D CAD view of the motor and tapper unit assembly present inside the micropump. Furthermore, the assembled micropump consisted of three components: (Figure 2a) the pump base, (Figure 2b) the tapping mechanism, and (Figure 2c) the tubing holder, which makes it modular. Parts such as the miniature DC motor and tapper head are precisely measured and then incorporated in the micropump design. The impedance micropump parts was designed on Google SketchUp version 2020, as shown in Figure 2. The created design was then imported in the stereolithography (.stl) format, an acceptable format for 3D printing. The final design after 3D printing resulted in a highly compact device of (22 mm × 10 mm × 11.7 mm) volume, including all the electromechanical components, excluding the microcontroller unit.
Figure 1.
3D designed CAD view of the motor and tapper unit assembly inside the proposed micropump with the Scotch and Yoke mechanism also known as the reciprocating mechanism that translates angular rotation (miniature DC motor) to linear movement (tapper head).
Figure 2.
3D designs produced on Google Sketchup 2020 showing the schematic view of (a) tapper head actuation slit (red); (b) sitting space for the pump and tubings (blue); (c) miniature motor socket(green); and (d) overall assembly with measurements.
The impedance pump is composed of an elastic and compressible tube unit made up of soft elastic material (i.e., PDMS) tubings, which are attached to both ends to a polytetrafluoroethylene (PTFE) tubing using resin-based glue. Although flexible, PTFE tubings are more rigid than PDMS, which is a desired characteristic of an impedance pump, as depicted by Figures 3 and 4a,b, after which the tubing assembly may then be fitted within the 3D printed impedance micropump body.
Figure 3.

All 3D printed parts with a small DC motor metal and a tapper head.
Figure 4.
(a) Fabricated PDMS elastic tubing; scale bar: 1 cm. (b) Impedance micropump tubing arrangement having an elastic segment attached at the center (PDMS tubing) with end-to-end coupling with rigid segments (PTFE tubings). Scale bar: 1 cm.
The main impedance pump comprises compressible elastic tubings (PDMS) and comparable rigid tubings (PTFE), as shown in Figure 4a. PTFE tubings were already purchased, and the compressible PDMS tubings were fabricated in the laboratory itself using a stainless-steel smooth wire with a cross-sectional diameter of 0.5 mm. PDMS with the standard 10:1 polymer to the curing agent ratio is prepared and left for at least 1 h at room temperature to initiate minor cross-linking. After 1 h, the stainless-steel wire is cut into 5 cm long pieces, dipped into the PDMS elastomer, and hung vertically in a hot air oven without turning it on for 20 min to remove the excess elastomer. 20 min later, the oven temperature is set to 100° and left for half an hour to cross-link the PDMS elastomer completely. Once the elastomer is cross-linked, the elastomer coating can be slipped off easily using chloroform, as shown in Figure 4a. The next step is to cut PDMS tubings in 1 cm of length and PTFE tubing in two pieces of 10 cm length each. The process aided us to fabricate PDMS elastic tubing of 0.5 mm internal diameter and 0.9 mm external diameter with only an average of ±5% variation in the outer/inner diameters of the aforesaid tubing. Additionally, the trivial variations in tubing diameters caused an insignificant alteration in the characteristic functioning of the impedance micropump. The PDMS tubing is attached end-to-end to the PTFE tubing via resin-based glue, as shown in Figure 4b, and the impedance micropump is ready for use.
Before designing the impedance micropump, excluding the external components such as a miniature DC motor and a tapper head, the precise measurements of all the parts were taken using a digital vernier caliper. 3D designing platform Google SketchUp version 2020 was used to design 3D parts of the micropump. Impedance micropump parts are designed into three parts. Once finished with the designing, the 3D (.stl) file needs to be exported and sliced using slicing software to obtain the 3D printable G.code file. A 3D DLP printer (model: Anycubic Photon Zero) was used to 3D print the designed parts using epoxy-based UV photocurable resin (AcryloCure) Figure 5d. All the parts were 3D printed in triplicate, and the copies were 3D printed all at once. Triplicate copies were 3D printed to assess the reproducibility and repeatability of the impedance micropump design. The DLP 3D printer has a UV LCD panel that can project the individual slice of the 3D created object to 3D print the desired design. UV photocurable resin is loaded into the resin vat. The 3D printer has an optically clear base to facilitate the light projections through the resin to the z-axis build plate. The UV projection instantly cures the projected region on the build plate immersed in the resin vat and increases with the set z-axis thickness, resulting in the precisely printed 3D object. The designed 3D impedance micropump is driven by linear tapping action of the mini tapper head, achieved by a reciprocating mechanism, also known as the Scotch and Yoke mechanism. For making a miniature reciprocating mechanism, the small motor shaft needs to have some eccentricity according to the desired linear displacement required. For this impedance micropump, a linear displacement of 0.5 mm is required, for which the shaft of the miniature motor is introduced with a minor bend and made it eccentric.
Figure 5.
Assembly of 3D printed parts serially: (a) all components of the microimpedance pump; (b) assembly of the pump base and tubing holder; and (c) integration of the tapping mechanism; scale bar: 2 cm. (d) DLP 3D printer used in the 3D printing of the all CAD designed parts using photocurable resin.
An 8 mm × 3 mm tapper head is fabricated using a thin 0.5 mm stainless-steel sheet with a slit of 1 mm × 0.5 mm at the center for inserting the eccentric shaft of the miniature motor to actuate it linearly.
After all the designed parts have been 3D printed, slight modifications of the miniature DC motor shaft are performed, and the main impedance micropump with proper tubings is ready. The next step is to assemble all the components into a single unit, as depicted by Figure 5a–c. The prepared micropump tubing is passed through the intrinsic beveled holes provided at both sides in the base part of the design to hold the tubings in the position. The tapping mechanism sits on the top of the base so that the tapper head aligns with the elastic compressible section and is slightly asymmetric from the center point of the flexible tube length. It was observed that the optimal tapping locating on the elastic section is found to be in the second half from the center. Before this, when tapped in the first and second halves, the pump is not able to reach the maximum pressure head. The rigid tube end near the tapper head becomes the outlet of the pump. A microcontroller board Arduino Mega 2560 is initially used to drive and test the assembled device. The miniature motor used in the impedance micropump operates on lower voltages (1–3 volts) consuming 120 mW of power and could easily be directly integrated with the microcontroller board. The input voltage in the small motor can be varied using a pulse width modulation (PWM) pin and a voltage controlling program on the microcontroller board.
Fabrication of the Prototype of Liver-On-Chip
The procedure for fabricating the liver-on-chip prototype was inspired by the method described by ref (20). The basic structure of the prototype consists of an upper channel and a lower channel separated by a porous membrane in between. This porous membrane is the most crucial part and acts as a conduit between the cells in the upper channel and the nutrient media flowing in the bottom channel. The whole liver-on-chip device is fabricated using a PDMS elastomer, which is chemically inert, non-toxic, and bio-compatible. The whole chip is a double layered structure which is aligned and bonded by placing a porous membrane composed of polyvinylidene difluoride (PVDF) in between the two chambers and bonding the whole device using corona discharge treatment creating two chambers, of which one is the cell chamber and the other one is the media flow chamber. The device is finally bonded over a glass substrate to provide mechanical strength and ease of handling to the device. Ten copies of the liver-on-chip device were fabricated initially after considering the experimental losses during the testes and to check the reproducibility and repeatability of the whole experimental setup including the liver-on-chip device efficiency. Two devices were sacrificed for the general durability tests, and the remaining eight devices were further used for the experimentation. Out of the remaining eight copies, three liver-on-chip devices were initially used for the testing with triplicate copies of the 3D printed impedance micropump.
Experimental Setup
The experimental setup consisted of the liver-on-chip integrated with the impedance micropump as shown in Figure 6. We have used a prototype of PDMS-based liver-on-chip microfluidic device to culture the HepG2 cells and test/validate the functionality of the proposed 3D impedance micropump.
Figure 6.
Culture medium flow circuit using an impedance micropump coupled to the liver-on-chip microfluidic chip via PTFE tubings (green) with flow controlled via an Arduino microcontroller board, while the microcontroller board is interfaced with the PC via (blue) cable. HepG2 cells present inside the liver-on-chip microfluidic chip being continuously circulated with the DMEM culture media.
The procedure for fabricating the liver-on-chip prototype was inspired by the method described by ref (20). The channels are first designed using AutoCAD 2020. The SU-8 master was fabricated through UV exposure. Two SU-8 masters with the same design but different depths were fabricated as the heights of the upper channel was greater than that of the lower channels. The basic structure of the prototype consists of an upper channel layer and a lower channel layer separated by a porous PVDF membrane in between with high porosity (total porosity ≥70%, mean pore size 0.2–0.45 μm). This porous membrane is the most crucial part and acts as a conduit between the cells in the upper channel and the nutrient media flowing in the bottom channel. Both these layers were casted using PDMS molds. The liver-on-chip prototype is designed in such a way that the cell chamber is separated via a porous membrane. The setup of the impedance micropump is connected to the lower media flow channel. Consequently, cells sit on the top of the membrane, and the nutrient media flowing in the bottom channel perfuses through the pores while maintaining their viability. The continuous flow is ensured by the impedance micropump integrated with the chip.
Cell Seeding and Culturing inside the Microchannels
The HepG2 cells were cultured following the standard protocol given by the manufacturer with all sterility measures to avoid contamination. The HepG2 cell line was procured from the National Cell Repository (NCSS, Pune, India). The HepG2 cells were cultured in a horizontal culture flask for 24 h in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic solution at 37 °C with 5% CO2. After 24 h, we observed 80–85% confluent cells. All microchannels were 0.1 mm tall and 0.3 mm wide. Downstream of the through holes, the channels were 15 mm long, yielding a constant 4.5 mm2 cell culture seeding area making up entire device volume of ∼1 μL. A confluence of 70–80% is required before seeding cells inside the device. The cell seeding density of (3 × 104 cells/mm2) was calculated according to the volume of the channels and the days of continuous incubation of cells in the device. The counting of cells before seeding was performed using a hemocytometer. The channels in the device were sterilized by flowing isopropyl alcohol (IPA) through the microchannels for 5 min, followed by washing with deionized (DI) water to remove the alcohol.
Type I collagen solution in acetic acid and sterile water was passed through the channels for 1 h to promote cell adhesion inside the channel. After this step, the microchannels were flushed with Hank’s balanced salt solution (HBSS, Sigma-Aldrich). Further, the device was placed under UV light for 5–10 min. The cells were seeded as per the total volume of the microchannel. The cells were allowed to attach to the collagen-coated microchannels in an incubator at 37 °C and 5% CO2. The device with the seeded cells is placed inside the incubator for 24 h. The cells are visualized under the microscope for cell confluency and adhesion. After 24 h, the cells were stained with a blue fluorescent nuclear dye Hoechst 33258 (Sigma-Aldrich, 0.25 μ g/mL), incubated for a minimum of 1 h at 37 °C, and visualized under a Nikon upright fluorescence microscope with water immersion objectives (model Evolution VF, Media Cybernetics, USA), and images were taken at 40× magnification. It was clearly visible that the cells were in good health and actively dividing.
Introduction of Flow Using an Impedance Pump
Further, the liver-on-chip was coupled in series with the impedance micropump in the closed-loop configuration for continuous perfusion study. As shown in Figure 6 earlier, the impedance micropump is coupled in series with the liver-on-chip microfluidic chip using extended PTFE tubings. External integration of the impedance micropump serves a series of purposes, providing ease of accessibility to decouple the liver-on-chip device from the setup for cell monitoring and imaging purpose and also eliminating any mechanical vibrations produced by the miniature motor, which may hamper the cell stability or may pollute the observations. The whole setup is then placed inside the CO2 incubator by properly covering it up in a sterile covering and then wiping the entire setup using 70% ethanol solution to avoid contamination. After every 10–15 h, fresh medium was introduced within the closed loop.
Results and Discussion
Flow Control
Flow control in the impedance micropump was achieved by varying the tapping frequency, as shown in Figure 1. Tapping frequency depends upon the RPM of the miniature motor, which is controlled electronically using a microcontroller interface for varying the tapping frequency, precisely providing the required voltage levels through modulation. The higher the voltage, the greater the RPM, and the higher the tapping frequency. Tapping frequency was measured and calculated using a small setup along with a microcontroller unit. As shown in Figure 7a, the upper part of the metal tapper unit is connected in the system such that it acts like a switch that closes and opens periodically, providing a series of pulsed outputs received at the analog input of the microcontroller programed to measure the delay in the incoming pulses, which is used to calculate the tapping frequency, while allowing the user to input voltage values to control the tapping frequency. Tapping frequency was measured with and without coupling with the impedance micropump, which affects the tapping frequency as shown in Figure 7b.
Figure 7.
Experimental setup schematic (a) for testing the tapping frequency of the tapper head where the extended part of the metallic tapper head connected with the signal input electrode acts as an open and close switch with a 3.3 V Vcc electrode while actuating and giving out a pulsed output, which is then used by the microcontroller to calculate the tapping frequency. (b) Plot for input voltage vs tapping frequency, showing varying tapping frequency, with and without coupling load configurations concerning the input voltage ranging from 0.2 to 3.0 V.
The flow rate was measured by collecting the fluid sample in marked PCR tubes and the time taken to fill the volume at different tapping frequencies. Upon studying the flow control in the impedance, the micropump flow initiates at around 10 Hz and reaches the maximum flow rate at around 35 Hz. At 40 Hz, the flow rate decreases and continues to decrease till 45 Hz. Upon reaching 50 Hz, the net flow rate becomes zero. The flow rate measurement was performed in triplicate. The readings were averaged, and the standard deviation was calculated. When increased further to around 55 Hz, a flow reversal in the opposite direction is observed with a negative flow rate, as depicted by Figure 8. The estimation of the flow properties was performed using two sample liquids: DI water and DMEM culture medium. The variation in the flow rates of both the sample liquids were observed because both the sample liquids exhibit different dynamic viscosities, which affects their flow properties.
Figure 8.

The graph shows the frequency flow response of the impedance micropump for variable tapping frequencies representing the forward (positive) and reverse (negative) flow. The black-colored plot represents the frequency flow response of the DI water when subjected to the variable tapping frequencies. The red-colored plot represents the frequency flow response of the DMEM culture media. The variation observed in the flow response represents the difference in the dynamic viscosity of both sample liquids.
Estimation of Pressure Generated during Operation
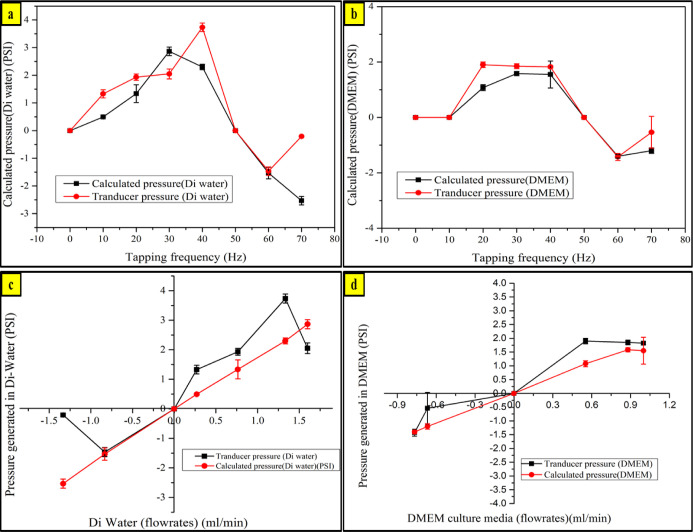
Pressure generated by the impedance micropump and pressure drop inside the liver-on-chip device are essential for the culture medium to pass through the microporous membrane over which the cells adhere. A pressure drop of a certain magnitude is required to push the culture medium through the micropores to reach out to the cell chamber. A pressure transducer is connected in between the impedance micropump and the liver-on-chip device for estimating the pressure drop across the liver-on-chip device. Pressure readings are taken at series of tapping frequencies, as shown in Figure 9. Pressure drop generated at variable frequencies were found to be sufficient enough to push the culture medium through the microchannels and through the porous membrane as well. While estimating pressure in the microfluidics domain dealing with laminar flow with alow Reynolds number, the relationship between the flow rate and pressure can be estimated from Hagen–Poiseuille’s law: ΔP = QRH where ΔP is the change in pressure in the microfluidic channel at a certain distance, Q is the volumetric flow rate of the fluid inside the channel, and RH is the hydraulic resistance presented by the microfluidic channel geometry. In devices being fabricated via photolithography are generally rectangular and with a depth generally less than the width of the microfluidic channel, for which RH can be estimated by RH = 12ηL/wh3, where η is the fluid viscosity, L is the channel length, w is the channel width, and h is the channel height. All the calculations were performed on the media flow chamber coupled with the impedance micropump with the following dimensions: L = 20 mm is the channel length, w = 1.3 mm is the channel width, and h = 0.15 mm is the channel height. The analogy of the system can be related with the Ohms law: ΔV = IR used for analyzing electronic circuitry.21
Figure 9.
Varying pressure inside the liver-on-chip with respect to the increasing tapping frequency of the tapping head compared with the calculated pressure vs the transducer pressure values tested with (a) DI water and (b) DMEM culture media. Varying pressure inside the liver-on-chip with respect to the varying flow rate tested with (c) DI water and (d) DMEM culture media.
A comparative study is performed by theoretically calculating the pressure values generated inside the liver-on-chip device, along with the pressure readings obtained using the pressure transducer. The pressure transducer is connected in between the impedance micropump and the liver-on-chip device while the proposed impedance micropump operates. Experiments were performed in triplicates, and the standard deviation was calculated for each case, as depicted by Figure 9. The study in Figure 9a,b shows the pressure variation inside the liver-on-chip device with respect to the tapping frequency of the tapping head for DI water and the DMEM culture medium, respectively. The study in Figure 9b,c reveals the linear relationship between the pressure generated and the flow rate for each case.
Impedance Micropump Integration with a Microfluidic Chip
The impedance micropump can be used to perform experiments where continuous perfusion and circulation studies are required. The impedance pump can operate in both open-loop and closed-loop configurations. Here, the pump has been used for perfusion studies performed on a microfluidic chip with the HepG2 human liver cancer cell line. The prepared culture medium is perfused and continuously circulated through the microfluidic chip containing cells. The study was performed and was monitored for 24 h at varying flow rates of 0.5, 1, 1.5, and 2 mL/min each. The experimental results showed a consistent standard deviation, as depicted in the graph. At the flow rate of 2 mL/min, we observed a large standard deviation. This indicates that 2 mL/min is the maximum limit of the impedance pump. This behavior is in accordance with the standard characteristic of an impedance pump. The impedance micropump is coupled with the lower microchannel in a closed-loop configuration. The medium is circulated and replaced after regular intervals. The whole setup of the closed-loop circulation consisting of the impedance micropump and the microfluidic chip is depicted in Figure 10a,b.
Figure 10.
Schematic diagram of (a) impedance micropump integration with the liver-on-chip device and pumping controlled using the Arduino microcontroller board and (b) cross-sectional view of the liver-on-chip microfluidic chip exposing the double layer structure of the device. The liver-on-chip device comprises two different chambers: the upper one is the cell chamber where the HepG2 cells were seeded and the other is the lower medium flow chamber, which is coupled with the impedance micropump for the continuous perfusion study.
Also, the closed-loop circuit flow eliminates any possible chances of cross-contamination. As PDMS is gas permeable, the chances of medium evaporation and drying of cells are quite high.22 This can be overcome by continuous medium circulation through the liver-on-chip microfluidic chip while maintaining an adequate medium and replenishing it with nutrients mimicking the natural blood flow conditions. A healthy cell population was observed inside the liver-on-chip microfluidic device. The impedance micropump provides a micro-pulsatile flow at a low tapping frequency of around 15–25 Hz. The optimal flow rate range is found to be 0.5–1.5 mL/min, in which cells inside the liver-on-chip microfluidic chip do not experience any shear stress during membrane deformation due to medium pressure developed inside the lower medium flow chamber with a wall shear stress of ∼38.9 dyn/cm2. On increasing the flow rate above 1.5 mL/min, the porous membrane starts to deform and the cells inside start experiencing a compressive force, resulting in permanent damage to the liver-on-chip device. After every 12 h, new medium was perfused while the impedance micropump is in the operational mode. Fresh medium was infused using a 1 mL syringe, pushing the used medium out of the circuit. Multiple liver-on-chip devices such as liver-on-chip, gut-on-chip, lung-on-chip, kidney-on-chip, and so forth,23−25 could be effectively integrated with the impedance micropump, which could be potentially used for high throughput screening and drug toxicity testing. While working for a microdomain flow application, the objective is to attain the desired flow rate and requirement of high pressure. The effect of gravity on flow almost nullifies as flow in the microfluidics domain remains laminar. The Reynolds number (Re) hardly exceeds 30, that is, R < 30. When the flow rate is the only aspect, impedance micropumping can be easily deployed as a simple, effective, miniature, and cost-effective solution.
Cell Viability Assay
Hoechst is a nuclear-specific blue fluorescent dye used to stain live or fixed cells. It is a non-toxic stable stain for staining live cells.
After subjecting the device to different flow rates, we stained the cells with a Hoechst 33342 stain to visualize how many cells survived inside the channel. This helped in estimating the optimal flow rate to keep the cells in a viable and adherent stage. In our experiment, the Hoechst 33342 dye was diluted to 10 μg/mL and added to the culture medium in the channel containing cells. The cells were incubated at cells at 37 °C for 1 h and then imaged under 40× magnification under a fluorescence microscope. Figure 11 shows the microscopic images of the cells inside the channels after being subjected to different flow rates. The maximum viability was observed at a flow rate of 1 mL/min, whereas cells were stable at lower flow rates. An AlamarBlue cell viability assay kit was purchased from Thermo Fisher Scientific, and a test was performed to confirm the viability of the HepG2 cells inside the liver-on-chip. The perfusion experiment was performed in triplicate at a flow rate of 1 mL/min. The AlamarBlue cell viability assay was performed. The assay showed maximum viability with respect to the control, as depicted by Figure 12. The viability of the cells inside the liver-on-chip depends upon the culture medium being fed to the cells after effectively passing through the membrane on which they are attached.
Figure 11.

(a) Microscopic image of HepG2 cells inside the liver-on-chip device obtained using an inverted microscope. (b–d) HepG2 cells stained with Hoechst dye maintained at flow rates of 0.5, 1.0, and 1.5 mL/min, respectively. Scale bar 20 μm.
Figure 12.

AlamarBlue cell viability assay at a flow rate pf 1 mL/min with respect to the control. After seeding the HepG2 cells inside the cell chamber of the liver-on-chip, the perfusion study was performed for 24 h, and the mean value of the cell viability data was calculated with respect to the control.
Conclusions
This work describes the design and development of an elegant simple valveless impedance micropump and a liver-on-chip prototype. It has been developed as an alternative to the conventional pumps and micropumps for OOC microfluidic chip-based applications. The experimental results at a specific flow rate show normal growth of HepG2 cells inside the OOC microfluidic chip while being connected to the impedance micropump, and no cellular detachment was observed inside the microfluidic chip while performing perfusion and circulation of culture media. Due to its light weight and small design, it can be easily placed along with the OOC microfluidic chip inside the incubator. Its modular design makes it is easy to assemble and repairable/replaceable in case of any issue with any of its constitutive components. Quite a few microfluidic biosensors modules may be directly interfaced with concurrent analyte samples via the proposed single impedance micropump for effective biosensing in an enclosed fluidic ambiance of microdevice and micropump. Owing to the easy manufacture procedure and its simple, cost-effective, minuscule, and closed modular blueprint, the projected micropump may be an efficient option as a peripheral pumping unit, for any lab-on-chip or in vitro BioMEMS application.26−31 Further, we are continuously making efforts to convert the abovementioned micropump into a fully automated entity with a wider range of flow rates, while maintaining its compact size.
Acknowledgments
Financial support from the Department of Science and Technology (DST) with grant no: DST/TM/WTI/2K15/201 and the Science and Engineering Board (SERB) with grant no: SR/FTP/ETA-0126/2014 is acknowledged. Support from the UGC-Start-up grant for Faculty Recharge Programme is recognized too. The Cell Culture Facility support from SCMM, JNU, New Delhi is also acknowledged.
Author Contributions
§ Amar Dhwaj and Nimisha Roy are equally contributing first authors.
The authors declare no competing financial interest.
References
- Jeong O. C.; Yang S. S. Fabrication and test of a thermopneumatic micropump with a corrugated p+ diaphragm. Sens. Actuators, A 2000, 83, 249–255. 10.1016/s0924-4247(99)00392-1. [DOI] [Google Scholar]
- Bao Q.; Zhang J.; Tang M.; Huang Z.; Lai L.; Huang J.; Wu C. A novel PZT pump with built-in compliant structures. Sensors 2019, 19, 1301. 10.3390/s19061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S.; Holloway P.; Batich C. D. Fabrication and testing of a magnetically actuated micropump. Sens. Actuators, B 2002, 87, 358–364. 10.1016/s0925-4005(02)00272-1. [DOI] [Google Scholar]
- Rinderknecht D.; Hickerson A. I.; Gharib M. A valveless micro impedance pump driven by electromagnetic actuation. J. Micromech. Microeng. 2005, 15, 861. 10.1088/0960-1317/15/4/026. [DOI] [Google Scholar]
- Prabhakar A.; Mukherji S. Microfabricated polymer chip with integrated U-bend waveguides for evanescent field absorption based detection. Lab Chip 2010, 10, 748–754. 10.1039/b921031h. [DOI] [PubMed] [Google Scholar]
- Prabhakar A.; Mukherji S. Investigation of the effect of curvature on sensitivity of bio/chemical sensors based on embedded polymer semicircular waveguides. Sens. Actuators, B 2012, 171–172, 1303–1311. 10.1016/j.snb.2012.05.013. [DOI] [Google Scholar]
- Prabhakar A.; Mishra N.; Mukherji S. A Comprehensive Investigation of a Microfabricated U-Bend Polymer Waveguide With Analyte Micro-Reservoir for Versatile On-Chip Sensing Applications. J. Microelectromech. Syst. 2017, 26, 935–945. 10.1109/jmems.2017.2697411. [DOI] [Google Scholar]
- Prabhakar A.; Mishra N.; Verma D.; Mukherji S. Investigation of dual-bend serpentine/spiral waveguides coupled to a microchannel system for competent, evanescent-wave-absorption-based, on-chip, biological-/chemical-sensing applications. RSC Adv. 2018, 8, 35539–35550. 10.1039/c8ra06527f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar A.; Mukherji S. A novel C-shaped, gold nanoparticle coated, embedded polymer waveguide for localized surface plasmon resonance based detection. Lab Chip 2010, 10, 3422–3425. 10.1039/c005253a. [DOI] [PubMed] [Google Scholar]
- Costa B. M. d. C.; Griveau S.; Bedioui F.; d’Orlye F.; da Silva J. A. F.; Varenne A. Stereolithography based 3D-printed microfluidic device with integrated electrochemical detection. Electrochim. Acta 2022, 407, 139888. 10.1016/j.electacta.2022.139888. [DOI] [Google Scholar]
- Hassan S.; Sebastian S.; Maharjan S.; Lesha A.; Carpenter A.-M.; Liu X.; Xie X.; Livermore C.; Zhang Y. S.; Zarrinpar A. Liver-on-a-chip models of fatty liver disease. Hepatology 2020, 71, 733. 10.1002/hep.31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low L. A.; Mummery C.; Berridge B. R.; Austin C. P.; Tagle D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discovery 2021, 20, 345–361. 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- Moradi E.; Jalili-Firoozinezhad S.; Solati-Hashjin M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 2020, 116, 67–83. 10.1016/j.actbio.2020.08.041. [DOI] [PubMed] [Google Scholar]
- Dongeum H.; Geraldine A.; Donald E. From three dimensional cell culture to organ on chips. Trends Cell Biol. 2011, 21, 745–754. 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luni C.; Serena E.; Elvassore N. Human-on-chip for therapy development and fundamental science. Curr. Opin. Biotechnol. 2014, 25, 45–50. 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Khot M. I.; Levenstein M. A.; de Boer G. N.; Maisey G.; Svavarsdottir T.; Andrew H. S.; Perry H.; Kapur S. L.; Jayne N.; Jayne D. G. Characterising a PDMS based 3D cell culturing microfluidic platform for screening chemotherapeutic drug cytotoxic activity. Sci. Rep. 2020, 10, 15915. 10.1038/s41598-020-72952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J.; Hwang G.; Arouche N.; Naserian S.; Harouri A.; Lotito V.; Casari C.; Lok T.; Menager J. B.; Issard J.; et al. A compact integrated microfluidic oxygenator with high gas exchange efficiency and compatibility for long-lasting endothelialization. Lab Chip 2021, 21, 4791. 10.1039/d1lc00356a. [DOI] [PubMed] [Google Scholar]
- Zislin V.; Rosenfeld M. Impedance pumping and resonance in a multi-vessel system. Bioengineering 2018, 5, 63. 10.3390/bioengineering5030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-H.; Tsai Y.-W.; Tsai C.-H.; Lee C.-Y.; Fu L.-M. Design and analysis of impedance pumps utilizing electromagnetic actuation. Sensors 2010, 10, 4040–4052. 10.3390/s100404040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D.; Kim H. J.; Fraser J. P.; Shea D. E.; Khan M.; Bahinski A.; Hamilton G. A.; Ingber D. E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- Behrens M. R.; Fuller H. C.; Swist E. R.; Wu J.; Islam M.; Long Z.; Ruder W. C.; Steward R.; et al. Open-source, 3D-printed peristaltic pumps for small volume point-of-care liquid handling. Sci. Rep. 2020, 10, 1543. 10.1038/s41598-020-58246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torino S.; Corrado B.; Iodice M.; Coppola G. Pdms-based microfluidic devices for cell culture. Inventions 2018, 3, 65. 10.3390/inventions3030065. [DOI] [Google Scholar]
- Huh D. A Human Breathing Lung-on-a-Chip. Ann. Am. Thorac. Soc. 2015, 12, S42–S44. 10.1513/annalsats.201410-442mg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No D. Y.; Lee J.; Lee S.-H.; et al. 3D liver models on a microplatform: well-defined culture, engineering of liver tissue and liver-on-a-chip. Lab Chip 2015, 15, 3822–3837. 10.1039/c5lc00611b. [DOI] [PubMed] [Google Scholar]
- Shim K.-Y.; Lee D.; Han J.; Nguyen N.-T.; Park S.; Sung J. H. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices 2017, 19, 37. 10.1007/s10544-017-0179-y. [DOI] [PubMed] [Google Scholar]
- Prabhakar A.; Agrawal M.; Mishra N.; Roy N.; Jaiswar A.; Dhwaj A.; Verma D. Cost-effective smart microfluidic device with immobilized silver nanoparticles and embedded UV-light sources for synergistic water disinfection effects. RSC Adv. 2020, 10, 17479–17485. 10.1039/d0ra00076k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N.; Kashyap J.; Verma D.; Tyagi R. K.; Prabhakar A. Prototype of a smart microfluidic platform for the evaluation of SARS-Cov-2 pathogenesis, along with estimation of the effectiveness of potential drug candidates and antigen–antibody interactions in convalescent plasma therapy. Trans. Indian Natl. Acad. Eng. 2020, 5, 241–250. 10.1007/s41403-020-00148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar A.; Verma D.; Dhwaj A.; Mukherji S. Microchannel integrated tapered and tapered-bend waveguides, for proficient, evanescent-field absorbance based, on-chip, chemical and biological sensing operations. Sens. Actuators, B 2021, 332, 129455. 10.1016/j.snb.2021.129455. [DOI] [Google Scholar]
- Prabhakar A.; Bansal I.; Jaiswar A.; Roy N.; Verma D. A simple cost-effective microfluidic platform for rapid synthesis of diverse metal nanoparticles: A novel approach towards fighting SARS-CoV-2. Mater. Today Proc. 2021, 10.1016/j.matpr.2021.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar A.; Verma D.; Roy N.; Nayak P.; Mukherji S.. Micro and Nanoelectronics Devices, Circuits and Systems; Springer, 2022; pp 291–305. [Google Scholar]
- Prabhakar A.; Jaiswar A.; Mishra N.; Kumar P.; Dhwaj A.; Nayak P.; Verma D. Amalgamation of diverse hydrodynamic effects with novel triple-sided membrane valves for developing a microfluidic device for filterless and continuous water purification. RSC Adv. 2021, 11, 28723–28734. 10.1039/d1ra04353f. [DOI] [PMC free article] [PubMed] [Google Scholar]