Figure 4. Effects of BicD2 inhibition on neuronal migration.
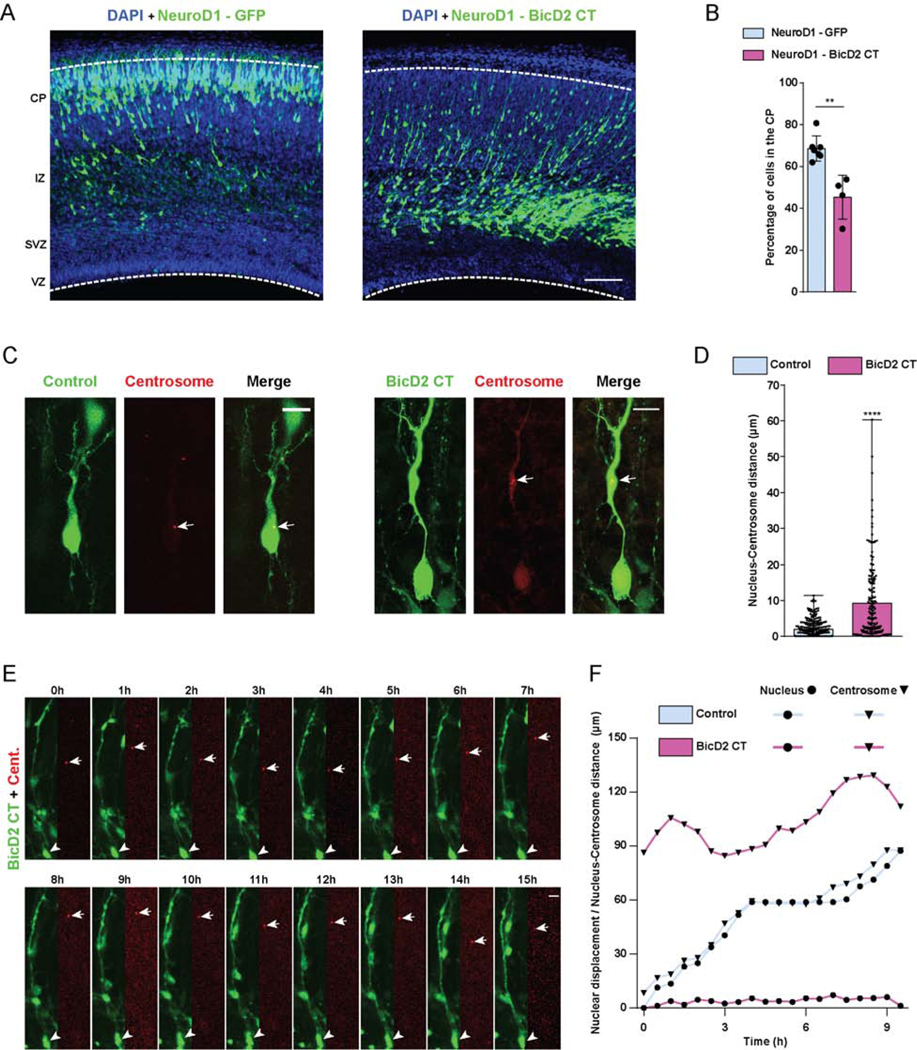
(A, B) E16 rat brain was electroporated with a cDNA encoding the BicD2 C-terminal domain under NeuroD1 promoter regulation for specific expression in post-mitotic neurons. Fixed brain slices were imaged at E20. (A) Representative images of the neocortex showing electroporated cells (green), co-stained with DAPI (blue). (B) Fraction of cells reaching the CP revealing that neuronal expression of BicD2-CT causes dramatic inhibition of neuronal migration. (C-F) E16 rat brain was electroporated with control GFP or the BicD2 CT fragment, along with PACT-DsRed, and imaged fixed at E20. (C) Representative images of electroporated neurons in the CP showing dramatically increased nucleus-centrosome spacing in BicD2-CT-expressing cells. (D) Quantification of N-C distance. (E) Time-lapse with dual-channel images for the soma and centrosome (red) are shown at 1h intervals (Video S4). Arrows and arrowheads indicate, respectively, centrosomes and cell bodies. (F) Graphical representation of relative position of the nucleus and centrosome over time. For each condition, nuclear displacement (●) was measured over time, relative to the original position (t=0). For each time point, N-C distance was quantified and summed to the nuclear position (▼). Data presented as mean±s.d. in B, and as scatter dot plot with bar representing median with range in D. Mann Whitney test for non-parametric distributions was used in B and D (**P<0.01; ****P<0.0001). Data in B include at least 3235 electroporated cells from at least 4 embryos, per condition. Data in D includes at least 169 electroporated cells from at least 4 embryos, per condition. A scale bar, 100μm. C and E scale bars, 10μm. Related to Figure S3 and Video S4.
