Abstract
Background
According to the International Rare Diseases Research Consortium (IRDiRC), a known rare disease (RD) should be diagnosable within a year. This study sought: firstly, to ascertain how long it takes to obtain the diagnosis of a RD in Spain, along with its associated time trend; and secondly, to identify and measure diagnostic delay (defined by the IRDiRC as any period exceeding a year) by reference to the characteristics of RDs and the persons affected by them.
Methods
Using data sourced from the Spanish Rare Diseases Patient Registry, we performed a descriptive analysis of the time elapsed between symptom onset and diagnosis of each RD, by sex, age and date of symptom onset, and type of RD. We analysed the time trend across the period 1960–2021 and possible change points, using a Joinpoint regression model and assuming a Poisson distribution. The multivariate analysis was completed with backward stepwise logistic regression.
Results
Detailed information was obtained on 3304 persons with RDs: 56.4% had experienced delay in diagnosis of their RDs, with the mean time taken being 6.18 years (median = 2; IQR 0.2–7.5). Both the percentage of patients with diagnostic delay and the average time to diagnosis underwent a significant reduction across the study period (p < 0.001). There was a higher percentage of diagnostic delays: in women (OR 1.25; 95% CI 1.07–1.45); in cases with symptom onset at age 30–44 years (OR 1.48; 95% CI 1.19–1.84): and when analysed by type of RD, in mental and behavioural disorders (OR 4.21; 95% CI 2.26–7.85), followed by RDs of the nervous system (OR 1.39; 95% CI 1.02–1.88).
Conclusions
This is the first study to quantify time to diagnosis of RDs in Spain, based on data from a national registry open to any RD. Since over half of all persons affected by RDs experience delay in diagnosis, new studies are needed to ascertain the factors associated with this delay and the implications this has on the lives of patients and their families.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-022-02530-3.
Keywords: Rare diseases, Diagnostic delay, Time to diagnosis, Patient registry, Spain, Public health
Background
Rare diseases (RDs) as a whole affect 3.5–8% of the population and thus represents a public health problem [1, 2]. Since these are very diverse diseases in terms of presentation and cause, obtaining the diagnosis of a RD in the shortest time possible poses a great challenge to health professionals and society. Despite there being increasingly more knowledge on the subject, more resources allocated and new technologies developed, diagnosis of most of these diseases continues to be complex. Hence, there is often a delay in diagnosis, which entails suffering for patients and their families alike, a high cost for the health system, as well as a possible exacerbation of the undiagnosed disease. Some of the causes of this delay in obtaining a diagnosis are linked to the lack of scientific knowledge on each of the thousands of RDs, the presence of nonspecific symptoms, the point in time when the patient seeks medical advice, the availability of diagnostic tests, and the difficulties facing specialists when it comes to making a complete diagnosis without waiting or losing time [3, 4].
Reducing diagnostic delay is a priority in research and socio-healthcare policies, as well as an ethical obligation. Among its goals for 2027, the International Rare Diseases Research Consortium (IRDiRC) requires that all known RDs be diagnosed within a maximum of one year from the date on which medical advice on the symptoms is first sought [5]. Hence, any case where a disease is diagnosed after more than a year will be deemed to qualify as a diagnostic delay.
Despite its importance, there is scant scientific evidence on delay in diagnosis of RDs [6], and what information is available on this topic tends to be furnished by patient associations. At a European level, EURORDIS indicates that “25% of patients had to wait between 5 and 30 years from early symptoms to confirmatory diagnosis of their disease. Also, 40% of patients first received an erroneous diagnosis, others received none” [7]. The Spanish Rare Disease Federation (Federación Española de Enfermedades Raras, FEDER) quantified the mean time taken by patients to obtain a diagnosis as 4 years, indicating that 20% took 10 years or more [8].
Both in the scientific world and in patient associations, the expression “diagnostic odyssey” [9, 10] is used to refer, not only to the delay in diagnosis, but also to this journey in which there may have been interminable referrals to specialists, interruptions in the search, or even erroneous diagnoses, at times amounting to a whole diagnostic process. Delay in diagnosis of RDs thus involves consequences, not only for patients but also for their families, such as receiving no support or treatment, or inappropriate treatment. This may in turn have repercussions, such as exacerbation of the disease, or effects on psychological [11], social [12], occupational [13], financial [14], educational [15], or family aspects [16], among others.
The aim of this study was thus: firstly, to ascertain the time taken to obtain the diagnosis of a RD in Spain, along with its associated time trend; and secondly, to characterise diagnostic delay on the basis of the IRDiRC goal, by reference to the main characteristics of RDs and the persons affected by them.
Methods
The data were drawn from the Rare Diseases Patient Registry at the Institute of Health Carlos III (Instituto de Salud Carlos III, ISCIII) [17]. The aim of the Registry was to provide researchers, health professionals and groups of patients with a greater level of knowledge on RDs, in order to foster research, increase these diseases’ visibility, and favour decision-making for appropriate healthcare planning and correct distribution of resources [18]. There are two ways in which entries can be made on this register: on the one hand, voluntarily, by the patients themselves or their families; and, on the other, by any professional participants in research networks or medical societies, with wide-ranging experience in a specific RD group, which have an agreement with the ISCIII to develop this record within the Rare Diseases Patient Registry. Patients registered are required to provide informed consent and clinical and/or genetic reports on their diagnoses, which are reviewed by the staff of the Institute of Rare Diseases Research (Instituto de Investigación de Enfermedades Raras, IIER), who then decide which RD is involved and code it accordingly. RDs are coded in the Registry in accordance with the respective classification systems, namely, ORPHAcode, International Classification of Diseases-Tenth Revision (ICD10) and, where possible, Online Mendelian Inheritance in Man (OMIM). If the information supplied does not suffice to specify the RD from which the patient suffers, the process is halted and new clinical reports are sought from the patient and/or his/her physician. When a suspected case of RD exists, the procedure of entry is the same than in confirmed ones: any patient can register voluntarily or any clinician can register a patient. In both cases, all the clinical information available should be provided to be studied. In addition, the Registry includes patient-reported data and questionnaires with different purposes, one of which is specifically targeted at the process of searching for the diagnosis of the RD. In this study, we included all patients entered on the Registry at 1 January 2022, who: had a confirmed diagnosis of a RD, along with detailed information about their diagnostic process; were of any age, sex or vital status (alive or deceased); and were Spanish residents at the moment of the enrolment in the registry.
The main variable of analysis -time to diagnosis- was calculated as the time elapsed (in years) from the date of symptom onset until the date when diagnosis of a RD was obtained. Based on the goal set by the IRDiRC, delay was defined as more than one year in this diagnosis process. In cases where the date of symptom onset or diagnosis was not available but the patient’s age at these points in time was in fact known, time to diagnosis was calculated on the basis of this information. In the case of prenatal diagnoses and pre-symptomatic diagnoses, diagnosis was assumed to have made at birth, so that the date of symptom onset and diagnosis coincided, and time to diagnosis was therefore 0 years.
We performed a descriptive analysis of the following variables of; sex;, patient’s age at symptom onset (under 15, 15–29, 30–44, and 45 years and over); decade of symptom onset (1960 to 2021); Autonomous Region “Comunidad Autónoma” (the 17 main regions on the basis of which public health services are organised in Spain); and type of RD (specific RDs, RD groups, or large groups in terms of the organ or principal system affected as per ICD10 criteria). See Additional file 1 to check the complete list of included RD and their ORPHAcodes.
For the time-trend analysis of the percentage of patients affected by diagnostic delay and the average time needed to achieve at a diagnosis, Annual Percent Changes (APC) were calculated using a Joinpoint regression model and assuming a Poisson distribution. The multivariate analysis was completed with a backward stepwise logistic regression, with likelihood ratio, in order to ascertain the odds ratio (OR) or risk of diagnostic delay according to the above-mentioned variables, taking the following as reference groups: men (sex); < 15 years of age (symptom onset); period 2010–2021 (decade of diagnosis); and endocrine, nutritional and metabolic diseases (type of RD). All analyses were performed using the SPSS v22 and Joinpoint v4.9.0.0 statistics programmes.
Results
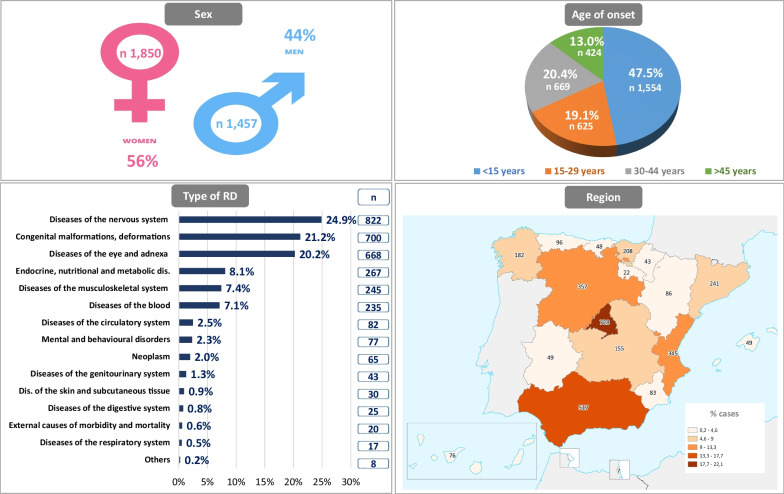
Information on time to diagnosis of RDs was obtained for 3,349 patients. Those cases with data that were incomplete or not in line with the natural history of the disease, were excluded. As a consequence of this screening process, the results reported below correspond to a total of 3,304 patients. Sample characteristics of patients included in this study are shown in Fig. 1.
Fig. 1.
Sample characteristics
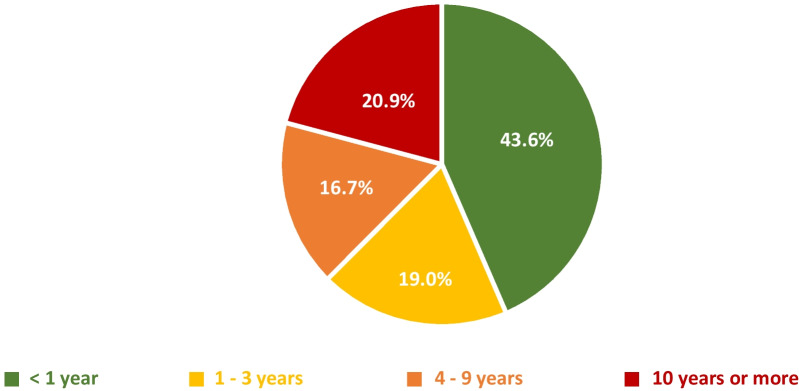
Mean time to diagnosis of a RD in Spain was 6.18 years (median = 2; IQR 0.2–7.5). More than half of all persons affected by RDs experienced some delay in the diagnosis of their disease (Fig. 2): 56.4% of patients took over a year, 19.0% took 1 to 3 years, 16.7% took 4 to 9 years, and 20.9% waited for more than 10 years to be diagnosed.
Fig. 2.
Time to diagnosis of RDs in Spain
Table 1 shows the percentage of persons who experienced a delay in diagnosis of their RD, by reference to examples of specific RDs or groups of RDs. Percentage delays of over 70% were observed in patients affected by diseases classified as mental and behavioural disorders, Usher syndrome, Sjögren’s syndrome, Behçet’s disease and cone-rod dystrophy. Special mention should be made of hereditary spastic paraplegias and post-polio syndrome which showed diagnostic delays in over 80% of patients.
Table 1.
Percentage of people who have suffered diagnostic delays in Spain, according to type of RD (classified according to the organ or system mainly affected), along with examples of specific RDs or RD groups represented by more than 30 cases
| Type of RD | RD or RD group | ICD10 | ORPHA code | n | Diagnostic delay (%) | ||
|---|---|---|---|---|---|---|---|
| Total | Men–Women | Total (%) | Men–Women (%) | ||||
| Diseases of the nervous system | Total | G00–G99 | 822 | 375–447 | 64.2 | 63.2–65.1 | |
| Tarlov cyst | G58.9 | 65, 250 | 128 | 14–114 | 57.8 | 64.3–57.0 | |
| Muscular dystrophies | G71.0 | 98, 473* | 90 | 52–38 | 67.8 | 59.6–78.9 | |
| Hereditary ataxias | G11 | 183, 518* | 74 | 40–34 | 63.5 | 67.5–58.8 | |
| Post-poliomyelitis syndrome | G14.0 | 2942 | 56 | 30–26 | 83.9 | 80.0–88.5 | |
| Hereditary spastic paraplegia | G11.4 | 685* | 53 | 33–20 | 88.7 | 84.8–95.0 | |
| Amyotrophic lateral sclerosis | G12.2 | 803 | 36 | 22–14 | 52.8 | 59.1–42.9 | |
| Myasthenia gravis | G70.0 | 589 | 35 | 15–20 | 34.3 | 20.0–45.0 | |
| Congenital malformations, deformations and chromosomal abnormalities | Total | Q00–Q99 | 700 | 308–392 | 48.4 | 45.8–50.5 | |
| Arnold-Chiari malformation | Q07.0 | 1136, 268, 882 | 60 | 19–41 | 65.0 | 52.6–70.7 | |
| Epidermolysis bullosa | Q81 | 304* | 38 | 20–18 | 44.7 | 40.0–50.0 | |
| Diseases of the eye and adnexa | Total | H00–H59 | 668 | 349–319 | 62.9 | 57.6–68.7 | |
| Retinitis pigmentosa | H35.5 | 791 | 384 | 185–199 | 65.1 | 61.1–68.8 | |
| Stargardt disease | H35.5 | 827 | 72 | 35–37 | 56.9 | 48.6–64.9 | |
| Usher syndrome | H35.5 | 886 | 55 | 32–23 | 76.4 | 71.9–82.6 | |
| Cone-rod dystrophy | H35.5 | 1872 | 30 | 16–14 | 70.0 | 68.8–71.4 | |
| Endocrine, nutritional and metabolic diseases | Total | E00–E90 | 267 | 131–136 | 55.4 | 48.9–61.8 | |
| Diseases of the musculoskeletal system and connective tissue | Total | M00–M99 | 245 | 76–169 | 53.1 | 42.1–58.0 | |
| Systemic sclerosis | M34 | 801* | 48 | 10–38 | 56.3 | 70.0–52.6 | |
| Behçet’s disease | M35.2 | 117 | 31 | 9–22 | 71.0 | 55.6–77.3 | |
| Sjögren-Larsson syndrome | M35.0 | 816 | 30 | 3–27 | 73.3 | 33.3–77.8 | |
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | Total | D50–D89 | 235 | 96–139 | 44.7 | 41.7–46.8 | |
| Hereditary angioedema | D84.1 | 91, 378* | 108 | 40–68 | 55.6 | 47.5–60.3 | |
| Sarcoidosis | D86 | 797 | 50 | 25–25 | 26.0 | 28.0–24.0 | |
| Diseases of the circulatory system | Total | I00–I99 | 82 | 10–72 | 51.2 | 30.0–54.2 | |
| Lymphangioleiomyomatosis | I89.8 | 538 | 46 | **− 46 | 56.5 | **− 56.5 | |
| Mental and behavioural disorders | Total | F00–F99 | 77 | 38–39 | 78.0 | 76.0–79.0 | |
| Neoplasms | Total | C00–D48 | 65 | 27–38 | 35.0 | 37.0–34.0 | |
| Diseases of the genitourinary system | Total | N00–N99 | 43 | 5–38 | 67.0 | 60.0–68.0 | |
| Diseases of the skin and subcutaneous tissue | Total | L00–L99 | 30 | 9–21 | 53.0 | 67.0–48.0 | |
| Total | 3304 | 1454-1850 | 56.4 | 53.4-58.8 | |||
*Group of disorders
**RDs that mainly affect women
In contrast, lower percentages of diagnostic delay were recorded by patients affected by congenital malformations, deformities and chromosomal anomalies, due to diseases of the blood and haematopoietic organs or due to epidermolysis bullosa (less than 50% of patients experienced a delay). Of note here were rare cancers, myasthenia gravis and sarcoidosis, with delays recorded in less than 35% of cases (Table 1).
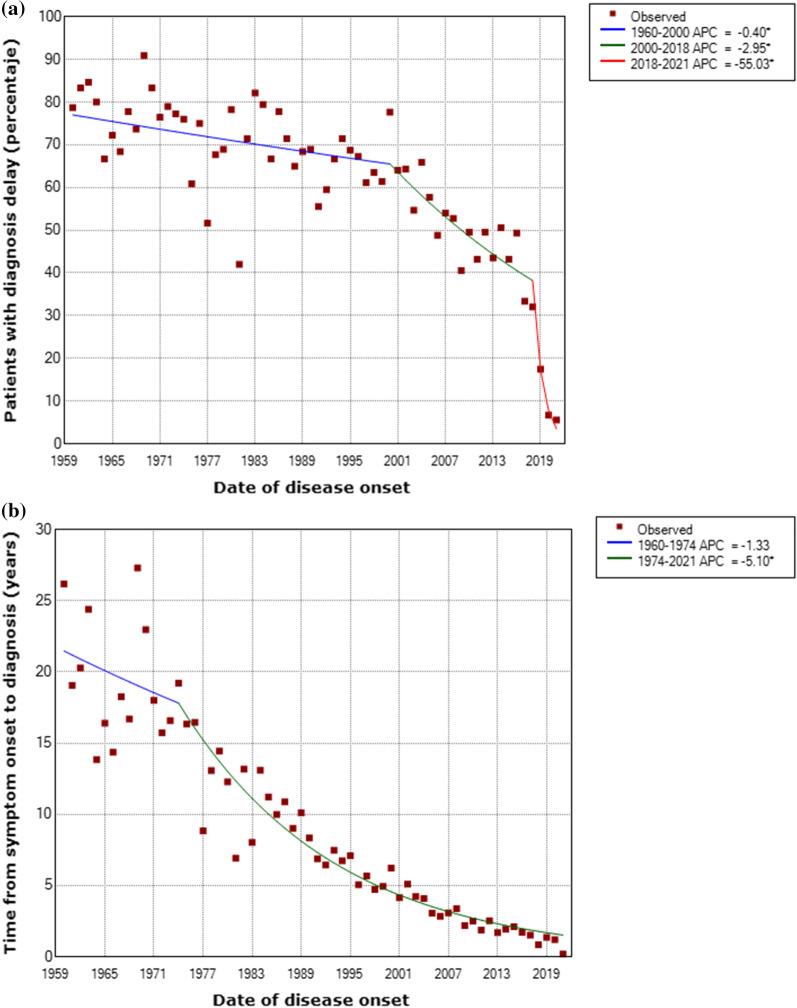
In terms of the time trend, the percentage of RD-affected patients who experienced diagnostic delays fell annually by 0.40% (APC) from 1969 until 2000 (p = 0.027), by 2.95% until 2018 (p < 0.001), and by even more from 2018 until 2021 (55.03%, p = 0.014; Fig. 3a). Similarly, time to diagnosis fell at an annual rate of 5.1% from 1974 to 2021 (p < 0.001; Fig. 3b).
Fig. 3.
Time trend in diagnostic delay (a) and time to diagnosis b of RDs in Spain 1960–2021. *Indicates that the Annual Percent Change (APC) is significantly different from zero at the alpha = 0.05 level. Note: Patients with symptom onset before 1960 were included in that year
When it comes to characterising diagnostic delays, Table 2 shows the results broken down by sex, age of symptom onset, decade of symptom onset, and type of disease (by ICD10 rubric). Greater diagnostic delays were observed among women (OR 1.25; 95% CI 1.07–1.45) and in cases where the age of symptom onset ranged from 30 to 44 years (OR 1.48; 95% CI 1.19–1.84). When RDs were broken down by organ or main system affected, mental and behavioural disorders (OR 4.21; 95% CI 2.26–7.85) and diseases of the nervous system (OR 1.39; 95% CI 1.02–1.88) had a higher risk of experiencing diagnostic delay. Compared to this, there was a lower risk of delay in diagnosis of rare cancers (OR 0.48; 95% CI 0.26–0.87), diseases of the blood, haematopoietic organs and other disorders that affect the immune mechanism (OR 0.64; 95% CI 0.44–0.94), and congenital malformations, deformities and chromosomal anomalies (OR 0.70; 95% CI 0.51–0.94). There were also differences according to decade of symptom onset, in that patients with symptom onset before 1979 had a fivefold higher risk of experiencing diagnostic delay (OR 5.27; 95% CI 4.00–6.94) than did those with symptom onset in the current period. The figures confirmed the fact that the more recent the date of symptom onset, the lower the percentage of patients affected by diagnostic delay. Lastly, it should be mentioned that no significant differences were found by patients’ home area or Autonomous Region (data not shown).
Table 2.
Time to diagnosis and diagnostic delays for RDs in Spain, based on characteristics of the disease and people affected
| Variable | Category | Time to diagnosis | Diagnostic delay (> 1 year) | |||
|---|---|---|---|---|---|---|
| n | Median (IQR) | Percentage | OR (95% CI) | p value | ||
| Sex | Men* | 1454 | 5.6 (0.1–6.3) | 53.4 | 0.005 | |
| Women | 1850 | 6.7 (0.2–8.5) | 58.8 | 1.25 (1.07–1.45) | 0.005 | |
| Age of onset of symptoms | < 15 years* | 1554 | 7.7 (0.1–10) | 55.2 | NS | |
| 15–29 years | 625 | 7.1 (0.2–11) | 61.8 | 1.19 (0.96–1.48) | NS | |
| 30–44 years | 669 | 4.1 (0.4–5.4) | 59.0 | 1.48 (1.19–1.84) | < 0.001 | |
| > 45 years | 424 | 2.6 (0.2–3.1) | 50.5 | 1.24 (0.96–1.60) | NS | |
| Decade of symptom onset | 2010–2021* | 969 | 0.9 (0.1–2.4) | 40.4 | < 0.001 | |
| 2000–2009 | 983 | 1.8 (0.2–5.7) | 56.5 | 1.95 (1.62–2.35) | ||
| 1990–1999 | 503 | 3.0 (0.3–11.0) | 65.0 | 2.90 (2.28–3.67) | ||
| 1980–1989 | 376 | 6.0 (0.4–19.0) | 69.7 | 4.60 (3.46–6.13) | ||
| Until 1979 | 431 | 13.1 (1–32.7) | 74.0 | 5.27 (4.00–6.94) | ||
| Type of RD | Endocrine, nutritional and metabolic diseases * | 267 | 6.1 (0.3–6.3) | 55.4 | < 0.001 | |
| Diseases of the nervous system | 822 | 6.3 (0.6–7.9) | 64.2 | 1.39 (1.02–1.88) | 0.035 | |
| Congenital malformations, deformations and chromosomal abnormalities | 700 | 5.8 (0–6.5) | 48.4 | 0.70 (0.51–0.94) | 0.016 | |
| Diseases of the eye and adnexa | 668 | 7.6 (0–10.0) | 62.9 | 1.03 (0.76–1.40) | NS | |
| Diseases of the musculoskeletal system and connective tissue | 245 | 4.6 (0.3–5.5) | 53.1 | 0.87 (0.60–1.27) | NS | |
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 235 | 6.5 (0–7.8) | 44.7 | 0.64 (0.44–0.94) | 0.021 | |
| Diseases of the circulatory system | 82 | 5.1 (0.1–4.9) | 51.2 | 0.70 (0.41–1.2) | NS | |
| Mental and behavioural disorders | 77 | 7.3 (1.2–10.9) | 77.9 | 4.21 (2.26–7.85) | < 0.001 | |
| Neoplasm | 65 | 4.1 (0.1–2.8) | 35.4 | 0.48 (0.26–0.87) | 0.015 | |
| Diseases of the genitourinary system | 43 | 6.3 (1–6.2) | 67.4 | 1.64 (0.80–3.34) | NS | |
| Diseases of the skin and subcutaneous tissue | 30 | 5.2 (0.1–7.8) | 53.3 | 0.80 (0.36–1.74) | NS | |
| Diseases of the digestive system | 25 | 2.1 (0–3.6) | 48.0 | 0.83 (0.35–1.97) | NS | |
| External causes of morbidity and mortality** | 20 | 0.1 (0–0.1) | 0.0 | 0.00 | NS | |
| Diseases of the respiratory system | 17 | 1.8 (0.2–2.5) | 41.2 | 0.78 (0.28–2.17) | NS | |
| Others | 8 | 5.6 (0.4–6.8) | 62.5 | 1.78 (0.40–7.88) | NS | |
*Reference groups
**People affected by Toxic oil syndrome, who were diagnosed less than one year from the onset of the epidemic in 1981
IQR interquartile range; OR odds ratio; NS no significant difference (p > 0.05)
Discussion
This is first study to quantify diagnostic delay for all RDs in Spain, based on information sourced from a national registry open to any RD, something that gives it an advantage over other studies based on surveys of patients with self-reported RDs. The reason for this is that all persons entered on the Spanish RD Patient Registry have a clinical report which makes it possible, on the one hand, to validate that they are indeed affected by a disease which fulfils the criteria for being considered a RD, and on the other, to verify their dates of symptom onset and diagnosis.
The mean time to diagnosis of a RD in Spain is 6.18 years, similar to that estimated by other international studies [19, 20]. More than half of all RD patients experience diagnostic delays in Spain (56.4%), with this being somewhat higher than the result obtained in the ENSERio study of FEDER [8], which reported a figure of 49.7% in a sample of 1576 patients. A more detailed comparison shows that both studies highlight the seriousness of this problem, with very similar percentages of diagnostic delay: 18.9% (ENSERio [8]) versus 19% (our study) of patients took 1 to 3 years, 18.1% versus 16.7% took 4 to 9 years, and 18.7% versus 20.9% waited more than 10 years to obtain a diagnosis. This thus confirms that for a high percentage of persons affected by RDs in Spain, the IRDiRC goal of obtaining diagnosis in the first year of examination has not be achieved.
In the international context, only EURORDIS’ EurordisCare 2 study offers general data for a group of 8 RDs, indicating that 25% of patients took more than 5 years to obtain their respective diagnoses [7]. Other studies report the median time to diagnosis for some RDs of the nervous system, i.e., Friedreich ataxia (36 months) [21], ataxia-telangiectasia (12 months) [22] and Duchenne muscular dystrophy (12 months) [23]. Furthermore, a time to diagnosis of less than a year has been reported for amyotrophic lateral sclerosis (11.5 months) [24] and inflammatory demyelinating polyradiculoneuropathy (5 months) [25]. In spite of the fact that there are diagnostic criteria for post-polio syndrome, 83.9% of these patients took over a year to be diagnosed (our study). Early diagnosis of this disease is complicated by the need for differential diagnosis, and even more so in the older population, by the overlapping of symptoms with other usual comorbidities. [26]
Similarly, time to diagnosis has also registered median values of over a year for some rare metabolic diseases: alpha-1 antitrypsin deficiency (22.3 years) [27], Pompe disease (12.9 years) [28], alpha-mannosidosis (72 months) [29], adult hypophosphatasia (over 3.8 years) [30], Farber’s disease (13.7 months) [31], and mucopolysaccharidosis type III (39 months) [32]. This delay in the diagnosis of rare metabolic diseases is more common among those that do not form part of neonatal screening or do not exhibit altered levels of biochemical parameters in routine biological samples [33]. In contrast, a time to diagnosis of less than a year is reported for cardiac amyloidosis (10 months) [34], mucopolysaccharidosis type I (9 months) [32] and childhood hypophosphatasia (8.4 weeks) [30].
In general, in addition to metabolic diseases which are diagnosed in neonatal screening, diseases of paediatric appearance (under age 15 years) are usually diagnosed before they present at adult age. For instance, at a paediatric level, congenital malformations predominate (39.1% of diseases of paediatric appearance), with the patients in question registering a 48.4% diagnostic delay in our study. Among adults, diseases that affect the nervous system are more frequent (35.8% of diseases of adult appearance), and patients in this group register a 64.2% delay. Regarding sex, differences in diagnostic delay have been found. Specifically, diagnostic delay is higher in women than in men in musculoskeletal, endocrine and eye and adnexa diseases. This gender gap is also observed in other studies with common diseases in which women are diagnoses later than men [35].
The difficulty of diagnosing certain RDs is due to the fact that many of them do not have specific tests and present with widely varying phenotypical manifestations [36]. In the case of RDs identified with diagnostic delay, it should be noted that such delay is especially harmful for patients affected by Usher Syndrome (our study indicates a diagnostic delay of over 75%), muscular dystrophies (Becker, Duchenne or facioescapulohumeral), Friedreich ataxia, lymphangioleiomyomatosis, hereditary angio-oedema, amyotrophic lateral sclerosis, or acquired epidermolysis bullosa. These RDs, among others, highlight the urgent need for development of solutions that would serve to reduce the delay in diagnosis [37].
In terms of time trend, the percentage of persons who experienced diagnostic delays decreased across the period between the 1960s and the present. This decrease is consistent with scientific and medical advances, increased knowledge of RDs, and improvements in diagnostic techniques. In this respect, the discovery of Next Generation Sequencing has radically changed diagnosis of certain genetic RDs, in that this is a fast, powerful and increasingly available alternative which has reduced waiting times [33]. The marked fall seen from 2018 onwards may be affected by the fact that all the study participants were persons already diagnosed with a RD, meaning that those with recent symptom onset have not yet had the opportunity to be diagnosed and included in this study. Given that mean time to diagnosis is 6 years, it would be more appropriate to pay attention to the trend registered until 2018, which shows a gradual decrease in the percentage of patients who experienced diagnostic delays in Spain. When it comes to Autonomous Regions, it should be stressed that while no evidence was found to show that diagnostic delays might vary by region, more in-depth study on this topic is nevertheless called for, since it is a matter of concern to patient associations.
It is clear that the diagnostic delay of RD is a public health problem. Therefore, it would be advisable to establish some potential measures like improving: the scientific knowledge of RDs; the accessibility to different clinicians o centres of references (sometimes by shortening the distance among them); the time spent in attending the different medical appointments; the availability of ad hoc diagnostic tests; the capacity of reffering to other centres easily or the increase of staff. In Spain there are 296 Reference Centres, Services and Units in the National Health System. They are distributed in 52 centres, includes 70 pathologies and many of them are part of the European Reference Networks. However, these centres do not cover all RD as many patients´ organizations claim.
Mention should be made of the difficulty of validating the variable, ‘time to diagnosis’. The limitations on being able to establish both disease onset and the fact that diagnosis was validly made are usual in these types of studies, if this information is not specifically recorded in the clinical report. If the first manifestations are clearly attributable to the disease, the date of symptom onset can be estimated, especially if it is connected to events in the family’s social life. The date of diagnosis can occur: (a) when it is linked to an analytical, histological or imaging test that allows for no doubt; or alternatively, (b) within the framework of a diagnostic process that is long per se. In such a case, diagnosis can be obtained with a high level of certainty in a given time, but confirmation may be altogether slower in coming, due to need to perform techniques that take time. Hence, whereas some patients might consider themselves to be already diagnosed on the basis of clinical suspicion, others would not venture to do so until receipt of the final confirmatory test, even though this may well corroborate the clinical diagnosis. These problems are further aggravated in the case of diagnoses made decades ago, though the general trend among patients insofar as it might serve to increase or decrease this time is not a systematic problem. In this study an effort was made to use the information stated in clinical reports, submitted by health professionals through medical societies, and/or recorded in the registry. Lastly, the lack of a population-based registry for RDs as a whole means that the distribution of all patients and all RDs in a specific geographical region cannot be properly ascertained. As a result, these aspects could limit extrapolation of the results obtained to RDs as whole.
Despite the limitations, the main strength of this study is that it is the first to quantify time to diagnosis of RDs in Spain, based on information sourced from a national registry open to any RD. Another point to be highlighted is that, in comparison with the only previous studies to furnish data on diagnostic delays in general for RDs in Spain [8, 38], our study not only relied on information covering a greater number of diseases and persons affected, confirmed diagnoses of RD, and a heterogeneous population in socio-demographic terms, but the data were also gathered over a long period of time.
Conclusion
This is the first study to quantify the time taken to arrive at a diagnosis, as well as diagnostic delays for RDs as a whole, based on the Spanish Rare Diseases Patient Registry. The data reported corroborate the fact that more than half of all persons affected by RDs in Spain experience diagnostic delays, and that, despite the reduction achieved since the 1960s, the country is still far from reaching the IRDiRC goal of obtaining a diagnosis within less than a year. It is therefore essential to continue monitoring time to diagnosis, while at the same time conducting in-depth studies to ascertain the factors associated with delay and the implications that all this has for every sphere of the lives of patients and their families, in view of the social and health problems involved.
Supplementary Information
Additional file 1. Table S1. Complete list of included RD and their ORPHAcodes (version 2021).
Acknowledgements
We thank all stakeholders who contribute to the Spanish Rare Diseases Patient Registry. First of all, patients and families for their willingness to sign up voluntarily and participate. Secondly, medical societies that collaborate with the Registry and also FEDER and CREER for encouraging patient participation. Finally, all the staff of the Institute of Rare Diseases Research, working in the Registry. We thank Michael Benedict for language editing.
Abbreviations
- APC
Annual percent change
- IRDiRC
International Rare Diseases Research Consortium
- RD
Rare diseases
- FEDER
Spanish Federation of Rare Diseases
- ISCIII
Institute of Health Carlos III
- IIER
Institute of Rare Diseases Research
- ICD
International Classification of Diseases
- IQR
Interquartile range
- NS
Non-significant difference
- OMIM
Online Mendelian Inheritance in Man
- OR
Odds ratio
Author contributions
JB participated in study design, data analysis, and drafted the manuscript. BL participated in data analysis and contributed to revising the manuscript. GA participated in the design of the study, in data analysis and contributed to revising the manuscript. MP participated in study design and contributed to revising the manuscript. VA participated in study design and contributed to writing and revising the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Spanish State Research Agency, State R&D Program Oriented to the Challenges of the Society, project no. RTI2018-094035-A-I00. JB enjoys a research grant funded by the Spanish Ministry of Science, Innovation and Universities (no. PRE2019-091508).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The Spanish Rare Diseases Patient Registry and the present study were approved by the Committee for Ethical Research of the Institute of Health Carlos III (CEI PI 74_2016 and CEI PI 58_2021-v2).
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juan Benito-Lozano, Email: jbenito@isciii.es.
Blanca López-Villalba, Email: blopezvi@saludcastillayleon.es.
Greta Arias-Merino, Email: garias@isciii.es.
Manuel Posada de la Paz, Email: mposada@isciii.es.
Verónica Alonso-Ferreira, Email: valonso@isciii.es.
References
- 1.Alonso V, Villaverde-Hueso A, Hens M, Morales-Piga A, Abaitua I, de la Posada PM. Public health research on rare diseases. Georgian Med News. 2011;193:11–6. [PubMed] [Google Scholar]
- 2.Wakap SN, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneu V, Murphy D, de Cam Y, Rath A. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28:165. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazal PE, Chalandon AS, Aymé S, Deleuze C. Diagnostic delay in rare diseases: a documented list of (296) rare diseases for which delayed diagnosis would be especially detrimental, based on the French situation. 2020. Preprint available at Research Square:10.21203/rs.3.rs-32308/v1.
- 4.Faviez C, Chen X, Garcelon N, Neuraz A, Knebelmann B, Salomon R, et al. Diagnosis support systems for rare diseases: a scoping review. Orphanet J Rare Dis. 2020;15(1):94. doi: 10.1186/s13023-020-01374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin CP, Cutillo CM, Lau LPL, et al. Future of rare diseases research 2017–2027: an IRDiRC perspective. Clin Transl Sci. 2018;11:21–27. doi: 10.1111/cts.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrocal-Acedo M, Benito-Lozano J, Alonso-Ferreira V, et al. Retraso diagnóstico en enfermedades raras: revisión sistemática. Rev Esp Salud Pública. 2022;96(10):e202201001. [PubMed] [Google Scholar]
- 7.Eurordis. EurordisCare2: survey of diagnostic delays, 8 diseases, Europe. 2007. https://www.eurordis.org/sites/default/files/publications/Fact_Sheet_Eurordiscare2.pdf. Accessed 16 Feb 2022.
- 8.FEDER, Federación Española de Enfermedades Raras. Estudio ENSERio. 2018. https://enfermedades-raras.org/index.php/estudio-enserio. Accessed 16 Feb 2022.
- 9.Black N, Martineau F, Manacorda T. Diagnostic Odyssey for rare diseases: exploration of potential indicators. Policy Innovation Research Unit. 2015. http://piru.lshtm.ac.uk/assets/files/Rare%20diseases%20Final%20report.pdf. Accessed 16 Feb 2022.
- 10.Kerr K, McAneney H, Smyth LJ, Bailie C, McKee S, McKnight AJ. A scoping review and proposed workflow for multi-omic rare disease research. Orphanet J Rare Dis. 2020;15:107. doi: 10.1186/s13023-020-01376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llubes-Arrià L, Sanromà-Ortíz M, Torné-Ruiz A, Carillo-Álvarez E, García-Expósito J, Roca J. Emotional experience of the diagnostic process of a rare disease and the perception of support systems: a scoping review. J Clin Nurs. 2021;31:20–31. doi: 10.1111/jocn.15922. [DOI] [PubMed] [Google Scholar]
- 12.Boettcher J, Boettcher M, Wiegand-Grefe S, Zapf H. Being the pillar for children with rare diseases—a systematic review on parental quality of life. Int J Environ Res Public Health. 2021;18:4993. doi: 10.3390/ijerph18094993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kole A, Faurisson F. Rare diseases social epidemiology: analysis of inequalities. Adv Exp Med Biol. 2010;686:223–250. doi: 10.1007/978-90-481-9485-8_14. [DOI] [PubMed] [Google Scholar]
- 14.Sequeira A, Mentzakis E, Archangelidi O, Paolucci F. The economic and health impact of rare diseases: a meta-analysis. Health Policy Technol. 2021 doi: 10.1016/j.hlpt.2021.02.002. [DOI] [Google Scholar]
- 15.Alfaro TM, Wijsenbeek MS, Powell P, et al. Educational aspects of rare and orphan lung diseases. Respir Res. 2021;22(1):92. doi: 10.1186/s12931-021-01676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinali P, Migliorini L, Rania N. The caregiving experiences of fathers and mothers of children with rare diseases in Italy: challenges and social support perceptions. Front Psychol. 2019;5(10):1780. doi: 10.3389/fpsyg.2019.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orden SCO/1730/2005, de 31 de mayo, por la que se crean y suprimen ficheros de datos de carácter personal gestionados por el Departamento. Boletín Oficial del Estado, 138, sec. III, de 10 de junio de 2005, 19987 a 19989. https://www.boe.es/eli/es/o/2005/05/31/sco1730. Accessed 16 Feb 2022.
- 18.Spanish Rare Diseases Patient Registry. Available at: https://registroraras.isciii.es/Comun/Inicio.aspx. Accessed 16 Feb 2022.
- 19.Choukair D, Hauck F, Bettendorf M, et al. An Integrated clinical pathway for diagnosis, treatment and care of rare diseases: model, operating procedures, and results of the project TRANSLATE-NAMSE funded by the German Federal Joint Committee. Orphanet J Rare Dis. 2021;16:474. doi: 10.1186/s13023-021-02092-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blöß S, Klemann C, Rother AK, Mehmecke S, Schumacher U, Mücke U, et al. Diagnostic needs for rare diseases and shared prediagnostic phenomena: results of a German-wide expert Delphi survey. PLoS ONE. 2017;12(2):e0172532. doi: 10.1371/journal.pone.0172532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Indelicato E, Nachbauer W, Eigentler A, Amprosi M, Matteucci R, Giunti P, et al. Onset features and time to diagnosis in Friedreich’s Ataxia. Orphanet J Rare Dis. 2020;15:198. doi: 10.1186/s13023-020-01475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devaney R, Pasalodos S, Suri M, Bush A, Bhatt JM. Ataxia telangiectasia: presentation and diagnostic delay. Arch Dis Child. 2017;102:328–330. doi: 10.1136/archdischild-2016-310477. [DOI] [PubMed] [Google Scholar]
- 23.D’Amico A, Catteruccia M, Baranello G, Politano L, Govoni A, Carlo-Previtali S, et al. Diagnosis of Duchenne muscular dystrophy in Italy in the last decade: critical issues and areas for improvements. Neuromuscul Disord. 2017;27:447–451. doi: 10.1016/j.nmd.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Palese F, Sartori A, Logroscino G, Pisa F. Predictors of diagnostic delay in amyotrophic lateral sclerosis: cohort study based on administrative and electronic medical records data. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3–4):176–185. doi: 10.1080/21678421.2018.1550517. [DOI] [PubMed] [Google Scholar]
- 25.Bunschoten C, Blomkwist-Markens P, Horemans A, van Doorn P, Jacobs B. Clinical factors, diagnostic delay, and residual deficits in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2019;24(3):253–259. doi: 10.1111/jns.12344. [DOI] [PubMed] [Google Scholar]
- 26.Amole M, Khouzam-Skelton N. Diagnosing Post-Polio syndrome in the elderly, a case report. Geriatrics. 2017;2(2):14. doi: 10.3390/geriatrics2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashenhurst JR, Nhan H, Shelton JF, Wu S, Tung JY, Elson SL, et al. Prevalence of alpha-1 antitrypsin deficiency, self-reported behavior change, and health care engagement among direct-to-consumer recipients of a personalized genetic risk report. Chest. 2022;161(2):373–381. doi: 10.1016/j.chest.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Vanherpe P, Fieuws S, D’Hont A, Bleyenheuft C, Demaerel P, De Bleecker J, et al. Late-onset Pompe disease (LOPD) in Belgium: clinical characteristics and outcome measures. Orphanet J Rare Dis. 2020;15(1):83. doi: 10.1186/s13023-020-01353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielonka M, Garbade S, Kölker S, Hoffmann G, Ries M. Ultra-orphan lysosomal storage diseases: a cross-sectional quantitative analysis of the natural history of alpha-mannosidosis. J Inherit Metab Dis. 2019;42:975–983. doi: 10.1002/jimd.12138. [DOI] [PubMed] [Google Scholar]
- 30.Högler W, Langman C, da Gomes Silva H, Fang S, Linglart A, Ozono K, et al. Diagnostic delay is common among patients with hypophosphatasia: initial findings from a longitudinal prospective global registry. BMC Musculoskelet Disord. 2019;20:80. doi: 10.1186/s12891-019-2420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielonka M, Garbade S, Kölker S, Hoffmann G, Ries M. A cross-sectional quantitative analysis of the natural history of Farber disease: an ultra-orphan condition with rheumatologic and neurological cardinal disease features. Genet Med. 2018;20(5):524–530. doi: 10.1038/gim.2017.133. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper GA, Meijer O, Langereis E, Wijburg F. Failure to shorten the diagnostic delay in two ultra-orphan diseases (mucopolysaccharidosis types I and III): potential causes and implications. Orphanet J Rare Dis. 2018;13:2. doi: 10.1186/s13023-017-0733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Marmiesse A, Gouveia S, Couce ML. NGS technologies as a turning point in rare disease research diagnosis and treatment. Curr Med Chem. 2018;25(3):404–432. doi: 10.2174/0929867324666170718101946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang D, Fournier P, Cariou E, Huart A, Ribes D, Cintas P, et al. Gateway and journey of patients with cardiac amyloidosis. ESC Heart Fail. 2020;7:2418–2430. doi: 10.1002/ehf2.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westergaard D, Moseley P, Sørup FKH, Baldi P, Brunak S. Population-wide analysis of differences in disease progression patterns in men and women. Nat Commun. 2019;10:666. doi: 10.1038/s41467-019-08475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaaf J, Sedlmayr M, Schaefer J, Storf H. Diagnosis of rare diseases: a scoping review of clinical decision support systems. Orphanet J Rare Dis. 2020;15(1):263. doi: 10.1186/s13023-020-01536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chazal PE, Aymé S. An objective approach to identify priority rare diseases for the development of solutions reducing the diagnostic delay based on french data. Front Pharmacol. 2021;12:734601. doi: 10.3389/fphar.2021.734601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benito-Lozano J, Arias-Merino G, Gómez-Martínez M, Ancochea-Díaz A, Aparicio-García A, Posada de la Paz M, Alonso-Ferreira V. Diagnostic process in rare diseases: determinants associated with diagnostic delay. Int J Environ Res Public Health. 2022;19(11):6456. doi: 10.3390/ijerph19116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Complete list of included RD and their ORPHAcodes (version 2021).
Data Availability Statement
Not applicable.