Abstract

An efficient [4 + 1] annulation reaction between in situ generated azoalkene intermediates and α-bromocarbonyls has been established. A series of skeletally diverse aza-heterocycles with a functionalized quaternary center were obtained in up to 89% yield under mild conditions.
Introduction
Dihydropyrazole and indoline skeletons are privileged units and widely exist in a number of natural products with a broad spectrum of biological activities.1 They have been employed as a receptor-interacting protein 1 kinase inhibitor, antibacterial agent, monoamine oxidase inhibitor, COX-2 inhibitor and acyl-CoA: cholesterol acyl-transferase (ACAT) inhibitor (Figure 1).2 Thus, an array of methods for the construction of such compounds have been developed.3 However, these protocols are often plagued by the need for metal catalysis and a high reactive partner to obtain an indoline-2-carboxylic acid skeleton and 4,5-dihydropyrazole-containing ester functional group. For instance, indoline-2-carboxylic acid skeletons were obtained through reduction of an indole derivative and cycloaddition reaction catalyzed by the metals Rh and Fe.3h,3i Meanwhile, the Nicolini group and Liu group reported a copper-catalyzed process with the combination of 1,2-diaza-1,3-diene and diazo ester providing ester-substituted dihydropyrazole.3l,3m Therefore, exploring a mild and metal-free method to achieve indoline-2-carboxylic acid and dihydropyrazole carboxylic acid skeletons is still desirable.
Figure 1.
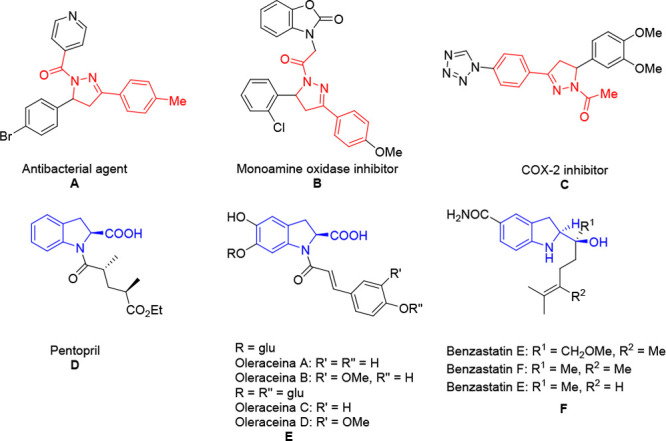
Biologically active molecules with a dihydropyrazole (A–C) and indoline skeleton (D–F).
The α-halo carbonyl compound has been proven as an attractive C1 partner for the construction of cyclopropane, oxirane, or other cyclic compounds.4 An N-containing five-membered compound could be obtained with an α-halo carbonyl compound as the C2 partner via the annulation reaction.5 Recently, the α-halo carbonyl was successfully used as the C3 partner to construct heterocyclic compounds with amine, an indole derivative, and alkene.6 However, α-halo carbonyl compounds are rarely employed in the [4 + 1] annulation of azoalkene due to the presence of other side reactions. Indeed, in 2019, Chen and co-workers developed a unique [4 + 1 + 1] annulation between α-bromo carbonyls and benzofuran-derived azadiene which offered the fused benzo-aza-heterocycle (Scheme 1a).7 Moreover, Zhao’s group used indanone-derived or benzofuran-derived azadiene as the starting material to react with bromomalonate, providing the cyclopropane product (Scheme 1b).8 A similar strategy was also used by Yang and co-workers to access spiro-cyclopropane indole derivatives (Scheme 1c).9 In all of the results mentioned above, the [4 + 1] annulation product could not be detected, which demonstrated the challenge of [4 + 1] annulation between azoalkene and α-halo carbonyl compounds. Due to our previous successes in the exploration of the transformation of azoalkene intermediates,10 we hypothesized that the [4 + 1] annulation would occur between the azoalkene intermediate and α-halo carbonyl compound, providing a metal- and organocatalysis-free protocol to obtain the functionalized dihydropyrazole and indoline derivative (Scheme 1d). Achievement of such a reaction is particularly challenging because: (1) the potential competing dimerization of the azoalkene intermediate leads to an eight-membered N-containing heterocycle;10b,11 (2) the construction of the quaternary carbon center in the product is difficult;12 and (3) the lower leaving ability of Br may suppress the occurrence of the [4 + 1] cycloaddition reaction.
Scheme 1. Annulation Pathways between Azoalkenes and α-Halo Carbonyl Compounds.

Results and Discussion
We initially treated α-halo hydrazone (2a) with KOH in the presence of diethyl 2-bromomalonate (1a) in CH2Cl2 at room temperature. To our delight, the [4 + 1] cycloaddition product dihydropyrazole 3a was isolated in 58% yield, which was identified through NMR data and confirmed by the X-ray crystallographic analysis of 3e.13 Then, several other bases were screened with the aim to improve the yield of cycloaddition product 3a. These results indicated that (1) all other screened bases were better than KOH except triethylamine (TEA) and triethylenediamine (TEDA) (Table 1, entries 1–8); (2) among the different bases, K2CO3 was proven to be the best one for this transformation (Table 1, entry 3); and (3) the type of base had an unignorable influence comparing the promotion effect of inorganic base and organic base on this reaction (Table 1, entries 7–8). Using K2CO3 as the base, various solvents have been evaluated. We found that this reaction in THF afforded 3a in 89% yield (Table 1, entry 9), while other solvents including CHCl3, MeCN, DMF, Et2O, and EtOAc were not as good as THF (Table 1, entries 10–14). Subsequent research showed that the temperature had little influence on the efficiency of this transformation (Table 1, entries 15–16). For example, elevated temperature to 70 °C led to the formation of 3a in 86% yield, while decreasing the temperature to 0 °C generated the product in 56% yield. Based on the above screening results, we thus obtained these optimal reaction conditions for this transformation: using K2CO3 as the base and THF as the solvent while stirring the reaction mixture at room temperature.
Table 1. Optimization of Reaction Conditionsa.
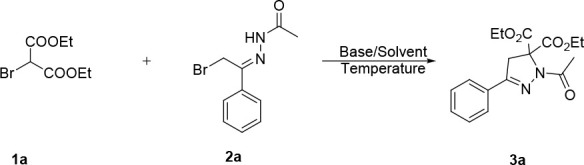
| entry | base | solvent | yield (%)b |
|---|---|---|---|
| 1 | KOH | CH2Cl2 | 58 |
| 2 | Cs2CO3 | CH2Cl2 | 62 |
| 3 | K2CO3 | CH2Cl2 | 81 |
| 4 | Na2CO3 | CH2Cl2 | 72 |
| 5 | NaHCO3 | CH2Cl2 | 75 |
| 6 | KOtBu | CH2Cl2 | 68 |
| 7 | TEDA | CH2Cl2 | trace |
| 8 | TEA | CH2Cl2 | trace |
| 9 | K2CO3 | THF | 89 |
| 10 | K2CO3 | CHCl3 | 76 |
| 11 | K2CO3 | MeCN | 77 |
| 12 | K2CO3 | DMF | 63 |
| 13 | K2CO3 | Et2O | 86 |
| 14 | K2CO3 | EtOAc | 43 |
| 15c | K2CO3 | THF | 56 |
| 16d | K2CO3 | THF | 86 |
Reaction conditions: 1a (0.15 mmol), 2a (0.1 mmol), and base (0.2 mmol) were reacted at room temperature.
Isolated yield based on 2a
Reaction was performed at 0 °C.
Reaction was performed at 70 °C.
With the optimized conditions in hand, the reaction compatibility was then explored, and these results were summarized in Table 2. The variation of α-halo hydrazone was tested first. When the azoalkene precursor bearing an electron-withdrawing group at the 4-position of the benzene ring was examined, each of the transformations underwent smooth, leading to the formation of the expected product in good yield (3b–3e). However, the fluorine group at the 4-position furnished the product 3f in lower yield. The possible reason is that the strong electron-withdrawing force affects the reactivity of the azoalkene intermediate. As anticipated, α-halo hydrazone having a 3-chlorophenyl group and a 3-bromophenyl group could also provide a similar cyclization process toward dihydropyrazole 3g and 3h in 81% and 85% yields, respectively. Delightedly, the 3,4-disubstituted azoalkene precursor was compatible in this annulation. Moreover, substrates containing an electron-donating (e.g., methyl) group at the para-position proved to be effective as well, which provided the corresponding dihydropyrazole 3j with comparable efficiency. In addition, the ether functional group was well-tolerated with this annulation system (3k). However, the tert-butyl-substituted azoalkene precursor seemed to be less reactive and achieved 3l in relatively lower yield. To our delight, a sterically encumbered naphthalen-2-yl counterpart was found to be a suitable partner, giving the product 3m in 80% yield. Next, we investigated the transformation of N-acetyl hydrazone with dimethyl malonate. These results indicated that aromatic-substituted, fused-ring-substituted, and alkyl-substituted hydrazones could be smoothly converted into their dihydropyrazole derivatives in 72–84% yields (3n, 3o, and 3p). Besides, other malonates such as dibenzyl 2-bromomalonate and diisopropyl 2-bromomalonate were all successfully tolerated and offered the expected products 3q and 3r. However, the yield was decreased to 54% when diisopropyl 2-bromomalonate was used as the starting material. We reasoned that the steric hindrance has a significant impact on this transformation. Notably, the C1 synthon is not restricted only to the malonate compound, but instead, the bromide of ethyl acetoacetate was also a suitable substrate for this transformation, and the corresponding annulation products (3s, 3t) were isolated in 83% and 60% yields, respectively. When 3-bromopentane-2,4-dione was used as the starting material, 3u was obtained in moderate yield.
Table 2. Scope with Various α-Halogeno Hydrazone and α-Bromocarbonyla.

Reaction conditions: 1 (0.15 mmol), 2 (0.1 mmol), and K2CO3 (0.2 mmol) were reacted at room temperature.
Isolated yield based on 2.
To further demonstrate the effectiveness of this method, we turned our attention to checking other azoalkene precursors such as 4a which was used as the four-unit intermediate. Thus, 4a was reacted with diethyl 2-bromomalonate 1a under the standard conditions. Delightedly, the desired annulation product 5a was observed in 26% yield. Next, we optimized the reaction conditions. A higher yield (65%) was obtained after the base screening (Table 3, entries 1–4). Subsequent solvent screening indicated it was more efficient when CH2Cl2 was used as the solvent, which delivered product 5a in 77% yield (entries 5–7). Finally, it was decided to carry out the cyclization process by using Cs2CO3 as the base and CH2Cl2 as the solvent.
Table 3. Optimization of Reaction Conditions for Indoline 5aa.
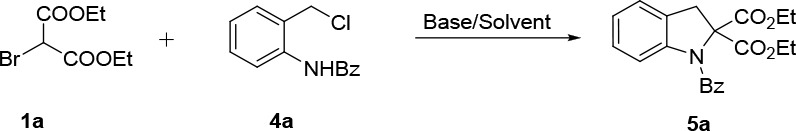
| entry | base | solvent | yield (%)b |
|---|---|---|---|
| 1 | K2CO3 | THF | 26 |
| 2 | Cs2CO3 | THF | 65 |
| 3 | KOtBu | THF | 31 |
| 4 | Na2CO3 | THF | 52 |
| 5 | Cs2CO3 | CH2Cl2 | 77 |
| 6 | Cs2CO3 | CH3CN | 74 |
| 7 | Cs2CO3 | Et2O | 48 |
Reaction conditions: 1a (0.15 mmol), 4a (0.1 mmol), and base (0.2 mmol) were reacted at room temperature.
Isolated yield based on 4a.
Next, the substrate scope of azoalkene precursor 4 was carefully studied under the optimized conditions. Some salient results were summarized in Table 4. Initially, we focused on the scope of the benzene ring. These results showed that the azoalkene precursor with electron-withdrawing property seemed to be more reactive. N-(2-(Chloromethyl) phenyl) benzamide 4 having F and CF3 substitutes at the 3-position were converted to the corresponding azoalkene intermediates which then underwent [4 + 1] annulation, affording the desired products in better yield (Table 4, 5b and 5c). Moreover, the electron-withdrawing groups at the 2-position and 4-position of the benzene ring were tolerated well and provided indoline derivatives 5d and 5e in moderate to good yield. The use of an electron-donating substituted azoalkene precursor offered the expected product 5f in good yield (63%) as well. Furthermore, a functionalized azoalkene intermediate with ether could also be used as the reaction partner to access the corresponding product 5g in 71% yield. The tert-butoxycarbonyl-protected azoalkene precursor was also used in this reaction, and the product 5a was obtained in 50% yield. In addition, the protecting group was not only limited to benzoyl (Bz) and tert-butoxycarbonyl (Boc). The annulation of N-benzyloxyformyl (Cbz) azoalkene precursors with C1 synthon 1a under the optimized conditions also furnished the corresponding products 5i–5k in 68–85% yields.
Table 4. Scope with the Azoalkene Precursor 4a,b.
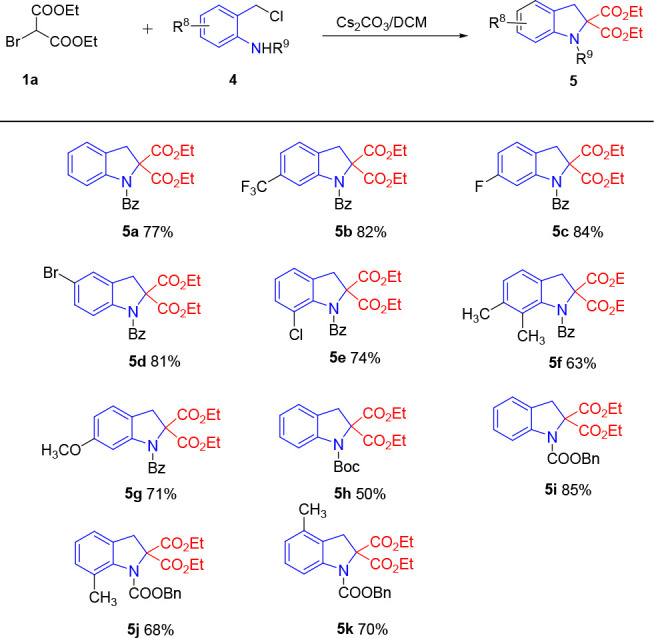
Reaction conditions: 1 (0.15 mmol), 4 (0.1 mmol), and Cs2CO3 (0.2 mmol) were reacted at room temperature.
Isolated yield based on 4.
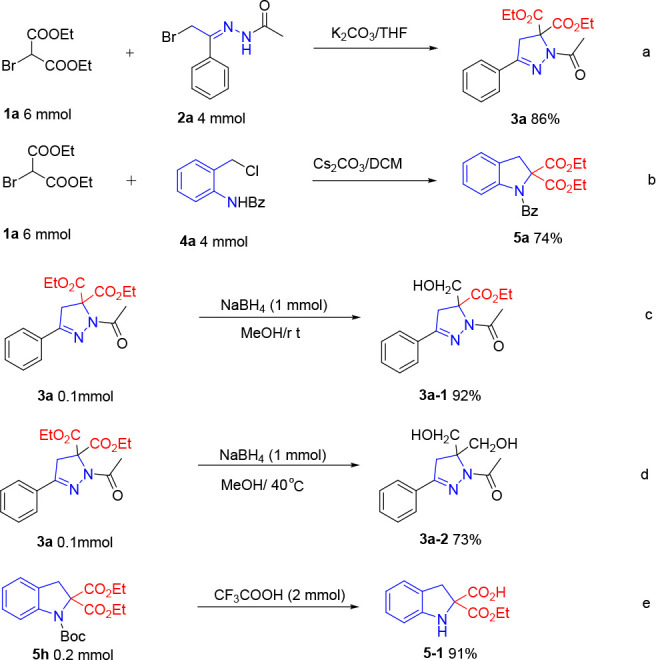
The synthetic utility of the annulation protocol was then demonstrated by performing these reactions on a gram scale, and these corresponding products 3a and 5a were isolated in 86% and 74% yield on a 4 mmol scale, respectively (Scheme 2a,b). Next, these late-stage transformations of product 3a were conducted. As shown in Scheme 2c,d, the selective reduction of dihydropyrazole 3a was realized with NaBH4 in CH3OH by controlling the temperature. 3a-1 was afforded in 92% yield at room temperature. While the reaction was run at 40 °C, the double reduction product with two hydroxyl groups 3a-2 was delivered in 73% yield (Scheme 2d). Besides, the protected indoline compound could offer the 2-carboxylate indoline derivative 5-1 under the catalysis of trifluoroacetic acid.
Scheme 2. Gram-Scale Reactions and the Transformation of 3a and 5h.
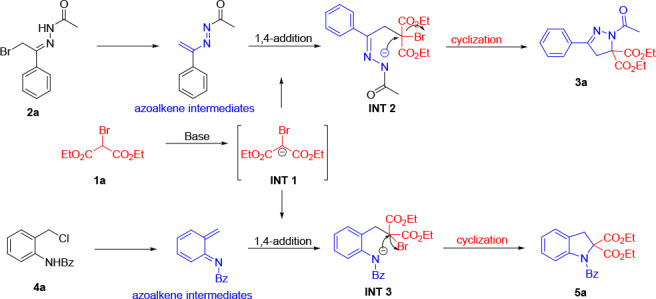
According to our previous studies and the literature,10 we proposed the following annulation mechanism (Scheme 3). These azoalkene intermediates were generated in situ via precursors 2a and 4a under the base conditions. Meanwhile, deprotonation of 1 formed the nucleophilic intermediate INT 1. Then, the 1,4-conjugate addition occurred between the INT 1 and azoalkene intermediates, offering INT 2 and INT 3. Subsequently, intramolecular cyclization through the simultaneous C–N and C–C bond formations offered [4 + 1] annulation products 3a and 5a.
Scheme 3. Proposed [4 + 1] Annulation Mechanism.
Conclusions
In summary, we have successfully developed a metal-free annulation protocol between α-bromocarbonyl compound 1 and α-halo hydrazone 2 or azoalkene precursor 4 to construct the skeletally diverse aza-heterocycle with a quaternary carbon center under mild conditions. Moreover, this approach has a general substrate scope for both the α-bromocarbonyl reagent and α-halo hydrazone or azoalkene precursor 4 with an electron-donating and electron-withdrawing group on the aromatic ring. Furthermore, this transformation developed a method for selective construction of a hydroxyl functional group in the dihydropyrazole compound.
Experimental Section
General Information
The 1H and 13C NMR spectra were recorded on a 400 MHz spectrometer with chloroform-d and dimethyl sulfoxide-d6 as the solvent. High-resolution mass spectra (HRMS) were recorded on an FT-ICR MS spectrometer. Melting points are uncorrected. Column chromatography was performed on silica gel (200–300 mesh). α-Bromocarbonyl compound 1 and azoalkene precursors 2 and 4 were synthesized according to literature methods.14
General Procedure for the Preparation of Dihydropyrazole 3
To a stirred solution of these α-bromocarbonyl compounds 1 (0.15 mmol) and K2CO3 (0.2 mmol) in THF (2 mL) at room temperature was added azoalkene precursor 2 (0.1 mmol). After the reaction was completed, as indicated by TLC, the mixture was concentrated in vacuo, and the crude product was purified by flash chromatography, eluting with ethyl acetate/petroleum ether = 1:20 to afford the product 3.
Diethyl 2-Acetyl-5-phenyl-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3a)
Ethyl acetate/petroleum ether = 1:20 was used as an eluent. White solid (30 mg, 89%). MP: 56.3–58.6 °C. 1H NMR (400 MHz, CDCl3): δ 7.74–7.66 (m, 2H), 7.45–7.37 (m, 3H), 4.32–4.25 (m, 4H), 3.83 (s, 2H), 2.43 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.06, 167.66, 151.94, 130.74, 130.47, 128.90, 126.70, 72.07, 62.95, 44.29, 21.92, 14.02. HRMS (ESI): m/z calcd for C17H21N2O5 [M + H]+: 333.1445, found 333.1449.
Diethyl 2-Acetyl-5-(4-cyanophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3b)
Ethyl acetate/petroleum ether = 1:15 was used as an eluent. White solid (31 mg, 87%). MP: 54.7–55.5 °C. 1H NMR (400 MHz, CDCl3): δ 7.79 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.4 Hz, 2H), 4.29 (qd, J = 7.2, 2.8 Hz, 4H), 3.81 (s, 2H), 2.43 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.14, 167.34, 149.91, 134.69, 132.63, 127.12, 118.33, 113.84, 72.41, 63.16, 43.86, 21.92, 14.00. HRMS (ESI): m/z calcd for C18H19N3NaO5 [M + Na]+: 380.1217, found 380.1218.
Diethyl 2-Acetyl-5-(4-chlorophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3c)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (31 mg, 85%). MP: 53.9–55.2 °C. 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 8.3 Hz, 2H), 7.39 (d, J = 8.3 Hz, 2H), 4.30 (dq, J = 7.1, 4.2 Hz, 4H), 3.80 (s, 2H), 2.42 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.94, 167.48, 150.72, 136.65, 129.10, 128.90, 127.84, 72.10, 62.94, 44.05, 21.82, 13.92. HRMS (ESI): m/z calcd for C17H19ClN2NaO5 [M + Na]+: 389.0875, found 389.0871.
Diethyl 2-Acetyl-5-(4-bromophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3d)
Ethyl acetate/petroleum ether = 1:20 was used as an eluent. White solid (36 mg, 88%). MP: 61.2–63.4 °C. 1H NMR (400 MHz, CDCl3): δ 7.55 (s, 4H), 4.30 (qd, J = 7.1, 2.7 Hz, 4H), 3.79 (s, 2H), 2.42 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.06, 167.59, 150.91, 132.18, 129.48, 128.16, 125.14, 72.24, 63.07, 44.13, 21.95, 14.05. HRMS (ESI): m/z calcd for C17H19BrN2NaO5 [M + Na]+: 433.0370, found 433.0378.
Diethyl 2-Acetyl-5-(4-nitrophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3e)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (31 mg, 82%). MP: 62.7–64.3 °C. 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.9 Hz, 2H), 7.85 (d, J = 8.9 Hz, 2H), 4.31 (qd, J = 7.2, 2.7 Hz, 4H), 3.85 (s, 2H), 2.45 (s, 3H), 1.32 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.20, 167.33, 149.58, 148.78, 136.48, 127.43, 124.19, 72.54, 63.23, 43.97, 21.96, 14.03. HRMS (ESI): m/z calcd for C17H19N3NaO7 [M + Na]+: 400.1115, found 400.1114.
Diethyl 2-Acetyl-5-(4-fluorophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3f)
Ethyl acetate/petroleum ether = 1:15 was used as an eluent. White solid (27 mg, 78%). MP: 48.9–51.7 °C. 1H NMR (400 MHz, CDCl3): δ 7.75–7.64 (m, 2H), 7.15–7.08 (m, 2H), 4.34–4.26 (m, 4H), 3.80 (s, 2H), 2.42 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.04, 167.67, 150.95, 128.78 (d, J = 8.0 Hz), 126.84, 116.16 (d, J = 21.0 Hz), 72.21, 63.05, 44.34, 21.95, 14.06. HRMS (ESI): m/z calcd for C17H19FN2NaO5 [M + Na]+: 373.1170, found 373.1172.
Diethyl 2-Acetyl-5-(3-chlorophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3g)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (30 mg, 81%). MP: 71.3–72.9 °C. 1H NMR (400 MHz, CDCl3): δ 7.70 (t, J = 1.8 Hz, 1H), 7.54 (dt, J = 7.5, 1.5 Hz, 1H), 7.42–7.32 (m, 2H), 4.29 (qd, J = 7.2, 3.0 Hz, 4H), 3.79 (s, 2H), 2.43 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.11, 167.50, 150.57, 135.02, 132.26, 130.64, 130.19, 126.66, 124.82, 72.18, 63.06, 44.11, 21.95, 14.03. HRMS (ESI): m/z calcd for C17H19ClN2NaO5 [M + Na]+: 389.0875, found 389.0871.
Diethyl 2-Acetyl-5-(3-bromophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3h)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (35 mg, 85%). MP: 51.9–54.1 °C. 1H NMR (400 MHz, CDCl3): δ 7.85 (t, J = 1.8 Hz, 1H), 7.62–7.54 (m, 2H), 7.29 (t, J = 7.9 Hz, 1H), 4.30 (qd, J = 7.1, 2.7 Hz, 4H), 3.79 (s, 2H), 2.43 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.14, 167.52, 150.46, 133.57, 132.56, 130.44, 129.61, 125.28, 123.10, 72.22, 63.08, 44.12, 21.97, 14.05. HRMS (ESI): m/z calcd for C17H19BrN2NaO5 [M + Na]+: 433.0370, found 433.0378.
Diethyl 2-Acetyl-5-(3,4-dichlorophenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3i)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent, White solid (33 mg, 83%). MP: 56.5–57.2 °C. 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 1.8 Hz, 1H), 7.54–7.47 (m, 2H), 4.30 (qd, J = 7.2, 2.9 Hz, 4H), 3.78 (s, 2H), 2.43 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.09, 167.44, 149.70, 134.82, 133.41, 130.96, 130.53, 128.42, 125.75, 72.33, 63.13, 44.01, 21.95, 14.04. HRMS (ESI): m/z calcd for C17H18Cl2N2NaO5 [M + Na]+: 423.0485, found 423.0481.
Diethyl 2-Acetyl-5-(p-tolyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3j)
Ethyl acetate/petroleum ether = 1:15 was used as an eluent. White solid (26 mg, 76%). MP: 61.3–64.6 °C. 1H NMR (400 MHz, CDCl3): δ 7.58 (d, J = 7.9 Hz, 2H), 7.22 (d, J = 7.9 Hz, 2H), 4.29 (qd, J = 7.2, 3.0 Hz, 4H), 3.81 (s, 2H), 2.43 (s, 3H), 2.39 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.02, 167.76, 152.06, 141.16, 129.61, 127.71, 126.68, 72.02, 62.94, 44.37, 21.95, 21.66, 14.05. HRMS (ESI): m/z calcd for C18H22N2NaO5 [M + Na]+: 369.1421, found 369.1423.
Diethyl 2-Acetyl-5-(4-methoxyphenyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3k)
Ethyl acetate/petroleum ether = 1:15 was used as an eluent, White solid (27 mg, 74%). MP: 57.4–58.8 °C. 1H NMR (400 MHz, CDCl3): δ 7.67–7.61 (m, 2H), 6.96–6.91 (m, 2H), 4.29 (qd, J = 7.2, 2.9 Hz, 4H), 3.85 (s, 3H), 3.80 (s, 2H), 2.42 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.93, 167.83, 161.67, 151.77, 128.38, 123.13, 114.34, 72.04, 62.94, 55.56, 44.41, 21.95, 14.06. HRMS (ESI): m/z calcd for C18H22N2NaO6 [M + Na]+: 385.1370, found 385.1366.
Diethyl 2-Acetyl-5-(tert-butyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3l)
Ethyl acetate/petroleum ether = 1:20 was used as an eluent. White solid (22 mg, 69%). MP: 59.6–61.1 °C. 1H NMR (400 MHz, CDCl3): δ 4.28 (ddt, J = 10.1, 7.1, 3.2 Hz, 4H), 3.41 (s, 2H), 2.31 (s, 3H), 1.29 (t, J = 7.1 Hz, 6H), 1.18 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.08, 167.94, 163.49, 72.13, 62.78, 43.65, 34.08, 27.99, 21.80, 14.07. HRMS (ESI): m/z calcd for C15H24N2NaO5 [M + Na]+: 335.1577, found 335.1576.
Diethyl 2-Acetyl-5-(naphthalen-2-yl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3m)
Ethyl acetate/petroleum ether = 1:20 was used as an eluent. White solid (31 mg, 80%). MP: 55.8–58.2 °C. 1H NMR (400 MHz, CDCl3): δ 8.00 (dd, J = 8.7, 1.7 Hz, 1H), 7.92 (s, 1H), 7.88–7.82 (m, 3H), 7.58–7.50 (m, 2H), 4.35–4.24 (m, 4H), 3.96 (s, 2H), 2.49 (s, 3H), 1.32 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.10, 167.72, 152.00, 134.36, 133.02, 128.77, 128.56, 128.10, 128.02, 127.58, 127.36, 127.00, 123.26, 72.20, 63.01, 44.29, 22.01, 14.07. HRMS (ESI): m/z calcd for C21H22N2NaO5 [M + Na]+: 405.1421, found 405.1422.
Dimethyl 2-Acetyl-5-phenyl-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3n)
Ethyl acetate/petroleum ether = 1:20 was used as an eluent. White solid (24 mg, 79%). MP: 50.8–51.9 °C. 1H NMR (400 MHz, CDCl3): δ 7.73–7.64 (m, 2H), 7.45–7.37 (m, 3H), 3.84 (s, 2H), 3.83 (s, 6H), 2.43 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.14, 168.19, 152.14, 130.82, 130.23, 128.89, 126.69, 71.75, 53.80, 44.43, 21.91. HRMS (ESI): m/z calcd for C15H16N2NaO5 [M + Na]+: 327.0951, found 327.0952.
Dimethyl 2-Acetyl-5-(naphthalen-2-yl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3o)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (29 mg, 84%). MP: 51.2–53.5 °C. 1H NMR (400 MHz, CDCl3): δ 7.99 (dd, J = 8.7, 1.7 Hz, 1H), 7.92 (s, 1H), 7.89–7.83 (m, 3H), 7.58–7.50 (m, 2H), 3.99 (s, 2H), 3.86 (s, 6H), 2.49 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.27, 168.26, 152.26, 134.36, 132.96, 128.79, 128.56, 128.00, 127.86, 127.63, 127.45, 127.01, 123.18, 71.89, 53.89, 44.44, 22.01. HRMS (ESI): m/z calcd for C19H18N2NaO5 [M + Na]+: 377.1108, found 377.1106.
Dimethyl 2-Acetyl-5-(tert-butyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3p)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (21 mg, 72%). MP: 50.6–52.3 °C. 1H NMR (400 MHz, CDCl3): δ 3.80 (s, 6H), 3.42 (s, 2H), 2.30 (s, 3H), 1.17 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.07, 168.50, 163.74, 71.75, 53.72, 43.73, 34.07, 27.95, 21.78. HRMS (ESI): m/z calcd for C13H20N2NaO5 [M + Na]+: 307.1264, found 307.1260.
Dibenzyl 2-Acetyl-5-(tert-butyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3q)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (33 mg, 76%). MP: 49.6–51.2 °C. 1H N MR (400 MHz, CDCl3): δ 7.31–7.21 (m, 10H), 5.19 (dd, J = 12, 2.3 Hz, 4H), 3.35 (s, 2H), 2.30 (s, 3H), 1.09 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3): δ 169.14, 167.51, 163.41, 135.05, 128.56, 128.39, 128.12, 72.00, 68.22, 43.58, 33.96, 27.81, 21.68. HRMS (ESI): m/z calcd for C25H28N2NaO5 [M + Na]+: 459.1890, found 459.1897.
Diisopropyl 2-Acetyl-5-(tert-butyl)-2,4-dihydro-3H-pyrazole-3,3-dicarboxylate (3r)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (19 mg, 54%). MP: 56.4–58.2 °C. 1H NMR (400 MHz, CDCl3): δ 5.07–5.01 (m, 2H), 3.32 (s, 2H), 2.24 (s, 3H), 1.21 (dd, J = 13.2, 6.3 Hz, 12H), 1.11 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.88, 167.11, 163.16, 72.26, 70.38, 43.34, 33.83, 27.71, 21.59, 21.51, 21.33. HRMS (ESI): m/z calcd for C17H28N2NaO5 [M + Na]+: 363.1890, found 363.1892.
Ethyl 1,5-Diacetyl-3-phenyl-4,5-dihydro-1H-pyrazole-5-carboxylate (3s)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (25 mg, 83%). MP: 64.2–65.6 °C. 1H NMR (400 MHz, CDCl3): δ 7.72–7.69 (m, 2H), 7.49–7.26 (m, 3H), 4.26 (qd, J = 7.2, 3.0 Hz, 2H), 3.91 (d, J = 17.6 Hz, 1H), 3.59 (d, J = 17.7 Hz, 1H), 2.46 (s, 3H), 2.45 (s, 3H), 1.30 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 130.92, 128.94, 126.82, 62.87, 43.17, 27.78, 22.04, 14.08. HRMS (ESI): m/z calcd for C16H18N2NaO4 [M + Na]+: 325.1159, found 325.1153.
Ethyl 1,5-Diacetyl-3-(tert-butyl)-4,5-dihydro-1H-pyrazole-5-carboxylate (3t)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (17 mg, 60%). MP: 62.5–63.2 °C. 1H NMR (400 MHz, CDCl3): δ 4.27 (qd, J = 10.7, 7.1 Hz, 2H), 3.52 (d, J = 17.7 Hz, 1H), 3.15 (d, J = 17.7 Hz, 1H), 2.40 (s, 3H), 2.32 (s, 3H), 1.28 (t, J = 7.1 Hz, 3H), 1.18 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3): δ 201.24, 169.34, 168.69, 164.90, 76.07, 62.63, 42.25, 34.13, 27.92, 27.67, 21.86, 14.03. HRMS (ESI): m/z calcd for C14H23N2O4 [M + H]+: 283.1652, found 283.1659.
1,1′,1″-(3-Phenyl-4,5-dihydro-1H-pyrazole-1,5,5-triyl)tris(ethan-1-one) (3u)
Ethyl acetate/petroleum ether = 1:15 was used as an eluent. White solid (21 mg, 77%). MP: 56.7–59.1 °C. 1H NMR (400 MHz, CDCl3): δ 7.70–7.64 (m, 2H), 7.44–7.36 (m, 3H), 3.60 (s, 2H), 2.44 (s, 3H), 2.27 (s, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 202.14, 169.87, 153.82, 131.06, 129.95, 128.88, 126.78, 81.06, 77.48, 77.16, 76.84, 41.64, 26.90, 22.00. HRMS (ESI): m/z calcd for C15H17N2O3 [M + H]+: 273.1234, found 273.1238.
General Procedure for the Preparation of Indoline 5
To a stirred solution of the α-bromocarbonyl compound 1 (0.15 mmol) and Cs2CO3 (0.2 mmol) in dichloromethane (DCM) (2 mL) at room temperature, azoalkene precursor 4 (0.1 mmol) was added. After the reaction was completed, as indicated by TLC, the mixture was concentrated in vacuo, and the crude product was purified by flash chromatography by eluting with ethyl acetate/petroleum ether = 1:10 to afford the product 5.
Diethyl 1-Benzoylindoline-2,2-dicarboxylate (5a)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (29 mg, 77%). MP: 123.1–125.2 °C. 1H NMR (400 MHz, CDCl3): δ 7.60–7.52 (m, 2H), 7.52–7.46 (m, 1H), 7.45–7.37 (m, 2H), 7.12 (d, J = 7.4 Hz, 1H), 6.88 (t, J = 7.4 Hz, 1H), 6.84–6.77 (m, 1H), 5.92 (d, J = 8.2 Hz, 1H), 4.26 (qd, J = 7.1, 2.7 Hz, 4H), 3.70 (s, 2H), 1.26 (t, J = 7.2 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.24, 167.85, 141.05, 135.40, 130.91, 128.66, 128.36, 127.34, 127.19, 124.84, 123.52, 114.68, 74.93, 62.30, 38.73, 13.84. HRMS (ESI): m/z calcd for C21H21NNaO5 [M + Na]+: 390.1312, found 390.1315.
Diethyl 1-Benzoyl-6-(trifluoromethyl) Indoline-2,2-dicarboxylate (5b)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (36 mg, 82%). MP: 127.9–129.4 °C. 1H NMR (400 MHz, CDCl3): δ 7.61–7.52 (m, 3H), 7.52–7.46 (m, 2H), 7.26 (d, J = 8.0 Hz, 1H), 7.18 (d, J = 7.8 Hz, 1H), 6.09 (s, 1H), 4.36–4.28 (m, 4H), 3.77 (s, 2H), 1.32 (td, J = 7.1, 1.0 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.58, 167.79, 141.84, 134.81, 132.49 (d, J = 1.3 Hz), 131.61, 129.98 (d, J = 32.3 Hz), 129.14, 127.41, 125.32, 123.56 (d, J = 272.4 Hz), 120.67 (q, J = 3.9 Hz), 111.78 (q, J = 4.2 Hz), 75.21, 62.86, 38.76, 14.05. HRMS (ESI): m/z calcd for C22H20F3NNaO5 [M + Na]+: 458.1186, found 458.1181.
Diethyl 1-Benzoyl-6-fluoroindoline-2,2-dicarboxylate (5c)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (33 mg, 84%). MP: 125.6–127.8 °C. 1H NMR (400 MHz, CDCl3): δ 7.60–7.52 (m, 3H), 7.49–7.44 (m, 2H), 7.08–7.04 (m, 1H), 6.61 (td, J = 8.5, 2.3 Hz, 1H), 5.62 (dd, J = 10.4, 2.2 Hz, 1H), 4.30 (qd, J = 7.1, 2.9 Hz, 4H), 3.66 (s, 2H), 1.29 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.54, 167.88, 162.03 (d, J = 243.7 Hz), 142.61 (d, J = 11.6 Hz), 135.01, 131.52, 129.07, 127.51, 125.64 (d, J = 9.9 Hz), 123.90 (d, J = 2.6 Hz), 110.34 (d, J = 23.0 Hz), 103.26 (d, J = 29.5 Hz), 75.89, 62.71, 38.27, 14.06. HRMS (ESI): m/z calcd for C21H20FNNaO5 [M + Na]+: 408.1218, found 408.1214.
Diethyl 1-Benzoyl-5-bromoindoline-2,2-dicarboxylate (5d)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent, White solid (36 mg, 81%). MP: 123.4–125.9 °C. 1H NMR (400 MHz, CDCl3): δ 7.59–7.51 (m, 3H), 7.47–7.42 (m, 2H), 7.29–7.27 (m, 1H), 6.97 (dd, J = 8.7, 2.1 Hz, 1H), 5.81 (d, J = 8.6 Hz, 1H), 4.31 (qd, J = 7.1, 3.4 Hz, 4H), 3.71 (s, 2H), 1.31 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.39, 167.80, 140.58, 135.11, 131.41, 130.86, 130.37, 129.02, 128.07, 127.62, 116.27, 116.25, 75.31, 62.79, 38.60, 14.08. HRMS (ESI): m/z calcd for C21H20BrNNaO5 [M + Na]+: 468.0417, found 468.0411.
Diethyl 1-Benzoyl-7-chloroindoline-2,2-dicarboxylate (5e)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (30 mg, 74%). MP: 126.5–128.7 °C. 1H NMR (400 MHz, CDCl3): δ 7.59–7.51 (m, 3H), 7.46 (dd, J = 8.7, 6.6 Hz, 2H), 7.04 (d, J = 8.0 Hz, 1H), 6.87 (dd, J = 8.0, 1.8 Hz, 1H), 5.85 (d, J = 1.8 Hz, 1H), 4.28 (qd, J = 7.1, 2.0 Hz, 4H), 3.65 (s, 2H), 1.28 (t, J = 7.2 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.45, 167.76, 142.39, 134.85, 133.02, 131.51, 129.01, 127.43, 126.99, 125.70, 123.65, 115.31, 75.47, 62.69, 38.39, 13.99. HRMS (ESI): m/z calcd for C21H20ClNNaO5 [M + Na]+: 424.0922, found 424.0926.
Diethyl 1-Benzoyl-6,7-dimethylindoline-2,2-dicarboxylate (5f)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (25 mg, 63%). MP: 121.7–123.5 °C. 1H NMR (400 MHz, CDCl3): δ 7.64–7.51 (m, 2H), 7.42 (t, J = 7.5 Hz, 1H), 7.33–7.26 (m, 2H), 6.97 (d, J = 7.5 Hz, 1H), 6.86 (d, J = 7.5 Hz, 1H), 4.29–4.15 (m, 4H), 3.81 (s, 2H), 2.04 (s, 3H), 1.40 (s, 3H), 1.24 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.39, 141.43, 137.23, 135.93, 131.37, 128.78, 128.21, 127.62, 126.53, 125.20, 121.53, 78.64, 62.56, 40.21, 20.00, 16.57, 13.96. HRMS (ESI): m/z calcd for C23H25NNaO5 [M + Na]+: 418.1625, found 418.1624.
Diethyl 1-Benzoyl-6-methoxyindoline-2,2-dicarboxylate (5g)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (28 mg, 71%). MP: 120.9–123.3 °C. 1H NMR (400 MHz, CDCl3): δ 7.62–7.56 (m, 2H), 7.56–7.43 (m, 3H), 7.01 (dd, J = 8.3, 1.0 Hz, 1H), 6.46 (dd, J = 8.3, 2.3 Hz, 1H), 5.49 (d, J = 2.2 Hz, 1H), 4.30 (qd, J = 7.2, 2.4 Hz, 4H), 3.64 (s, 2H), 3.38 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.71, 152.51, 141.61, 135.49, 133.64, 128.61, 128.50, 128.36, 125.02, 124.54, 112.72, 67.73, 62.50, 39.38, 18.49, 13.89. HRMS (ESI): m/z calcd for C22H23NNaO6 [M+ Na]+: 420.1418, found 420.1422.
1-(tert-Butyl) 2,2-Diethyl Indoline-1,2,2-tricarboxylate (5h)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (18 mg, 50%). MP: 128.7–129.9 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.80 (s, 1H), 7.26–7.15 (m, 2H), 6.99 (t, J = 7.4 Hz, 1H), 4.19 (q, J = 7.2 Hz, 4H), 3.62 (s, 2H), 1.40 (s, 9H), 1.20 (t, J = 7.3 Hz, 6H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 168.39, 128.34, 124.93, 123.35, 114.48, 81.98, 73.39, 62.46, 27.96, 14.26. HRMS (ESI): m/z calcd for C19H25NNaO6 [M + Na]+: 386.1574, found 386.1569.
1-Benzyl 2,2-Diethyl Indoline-1,2,2-tricarboxylate (5i)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (34 mg, 85%). MP: 106.3–107.4 °C. 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 8.2 Hz, 1H), 7.52–7.09 (m, 7H), 6.98 (d, J = 7.8 Hz, 1H), 5.28 (d, J = 60.2 Hz, 2H), 4.28–3.96 (m, 4H), 3.69 (s, 2H), 1.26–1.02 (m, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.49, 162.31, 152.43, 141.80, 135.40, 128.44, 128.30, 128.18, 126.15, 124.01, 123.35, 115.17, 73.81, 67.70, 62.41, 40.16, 13.79. HRMS (ESI): m/z calcd for C22H23NNaO6 [M + H]+: 398.1598, found 398.1591.
1-Benzyl 2,2-Diethyl 7-Methylindoline-1,2,2-tricarboxylate (5j)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (25 mg, 60%). MP: 99.7–102.1 °C. 1H NMR (400 MHz, CDCl3): δ 7.42–7.31 (m, 5H), 7.05–6.96 (m, 3H), 5.24 (s, 2H), 4.11 (qq, J = 7.3, 3.6 Hz, 4H), 3.77 (s, 2H), 2.27 (s, 3H), 1.18 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.63, 152.26, 139.69, 135.53, 131.01, 129.22, 128.46, 128.24, 128.18, 126.90, 124.62, 121.46, 75.95, 67.79, 62.48, 40.30, 20.76, 13.86. HRMS (ESI): m/z calcd for C23H25NNaO6 [M + Na]+: 434.1574, found 434.1578.
1-Benzyl 2,2-Diethyl 4-Methylindoline-1,2,2-tricarboxylate (5k)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (29 mg, 70%). MP: 97.3–98.8 °C. 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 8.1 Hz, 1H), 7.50–7.31 (m, 5H), 7.21–7.08 (m, 1H), 6.85 (d, J = 7.8 Hz, 1H), 5.41–5.18 (m, 2H), 4.30–3.98 (m, 4H), 3.63 (s, 2H), 2.21 (s, 3H), 1.30–1.08 (m, 6H). 13C{1H} NMR (100 MHz, CDCl3): δ 168.48, 168.15, 159.18, 142.38, 135.68, 131.12, 128.94, 127.69, 125.29, 120.45, 109.82, 101.51, 75.88, 62.62, 55.10, 38.34, 29.82, 14.12. HRMS (ESI): m/z calcd for C23H25NNaO6 [M + Na]+: 434.1574, found 434.1577.
General Procedure for the Preparation of 3a-1
To a stirred solution of the compound 3a (0.1 mmol) in MeOH (2 mL) at room temperature was added NaBH4 (1 mmol). After the reaction was completed as indicated by the TLC, the mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with ethyl acetate/petroleum ether = 1:10 to afford the products 3a-1 in 92% yield.
Ethyl 1-Acetyl-5-(hydroxymethyl)-3-phenyl-4,5-dihydro-1H-pyrazole-5-carboxylate (3a-1)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. White solid (27 mg, 92%). MP: 93.4–96.5 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.78–7.71 (m, 2H), 7.50–7.43 (m, 3H), 5.12 (t, J = 7.8 Hz, 1H), 4.18 (dd, J = 11.5, 5.7 Hz, 1H), 4.14–4.05 (m, 2H), 3.69 (dd, J = 11.5, 6.8 Hz, 1H), 3.63–3.48 (m, 2H), 2.27 (s, 3H), 1.13 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 170.32, 168.24, 152.77, 131.05, 130.75, 129.23, 126.90, 70.19, 61.33, 61.05, 41.86, 22.23, 14.33. HRMS (ESI): m/z calcd for C15H18N2NaO4 [M + Na]+: 313.1159, found 313.1158.
General Procedure for the Preparation of 3a-2
To a stirred solution of the compound 3a (0.1 mmol) in MeOH (2 mL) at room temperature was added NaBH4 (1 mmol). Then, the mixture was heated to 40 °C. After the reaction was completed an indicated by TLC, the mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with ethyl acetate/petroleum ether = 1:4 to afford the product 3a-2 in 73% yield.
1-(5,5-Bis(hydroxymethyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl) ethan-1-one (3a-2)
Ethyl acetate/petroleum ether = 1:4 was used as an eluent. White solid (18 mg, 73%). MP: 112.4–114.6 °C. 1H NMR (400 MHz, DMSO-d6): δ 7.74–7.70 (m, 2H), 7.48–7.42 (m, 3H), 4.99 (s, 2H), 3.98 (d, J = 11.0 Hz, 2H), 3.52 (d, J = 11.0 Hz, 2H), 3.34 (s, 2H), 2.25 (s, 3H). 13C{1H} NMR (100 MHz, DMSO-d6): δ 168.87, 152.87, 131.55, 129.96, 128.76, 126.27, 72.80, 61.04, 23.16. HRMS (ESI): m/z calcd for C13H16N2NaO3 [M + Na]+: 271.1053, found 271.1055.
General Procedure for the Preparation of 5-1
To a stirred solution of compound 5h (0.2 mmol) in dichloromethane (2 mL) at 0 °C was added trifluoroacetic acid (TFA) (2 mmol). Then, the mixture was heated to 30 °C. After the reaction was completed, as indicated by TLC, the mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with ethyl acetate/petroleum ether = 1:10 to afford the product 5-1 in 91% yield.
2-(Ethoxycarbonyl) Indoline-2-carboxylic Acid (5-1)
Ethyl acetate/petroleum ether = 1:10 was used as an eluent. Colorless oil liquid (43 mg, 91%). 1H NMR (400 MHz, CDCl3): δ 9.15 (s, 1H), 7.26 (td, J = 7.7, 1.4 Hz, 1H), 7.20 (d, J = 7.5 Hz, 1H), 7.08 (td, J = 7.5, 1.1 Hz, 1H), 6.92 (dd, J = 7.9, 1.2 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 3.97 (d, J = 16.6 Hz, 1H), 3.40 (d, J = 16.6 Hz, 1H), 1.31 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3): δ 166.56, 165.13, 135.92, 128.50, 128.29, 124.19, 120.88, 115.95, 63.80, 58.50, 39.90, 14.05. HRMS (ESI): m/z calcd for C12H13NNaO4 [M + Na]+: 258.0737, found 258.0739.
Acknowledgments
We are grateful for financial support from the Youth Science & Technology Personal Growth Project of the Educational Department of Guizhou Province (KY [2021] 227), the Guizhou Province Science and Technology plan program of China (QKHPTRC [2019]-034), CK-1187-029 and Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2020FYYX189).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04127.
1H and 13C{1H} NMR spectra for all of the products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Silva V. L. M.; Elguero J.; Silva A. M. S. Current progress on antioxidants incorporating the pyrazole core. Eur. J. Med. Chem. 2018, 156, 394. 10.1016/j.ejmech.2018.07.007. [DOI] [PubMed] [Google Scholar]; b Korablina D. D.; Vorozhtsov N. I.; Sviridova L. A.; Kalenikova E. I.; Medvedev O. S. Pharmacological Activity of 4,5-Dihydropyrazole Derivatives. Pharm. Chem. J. 2016, 50, 281. 10.1007/s11094-016-1438-6. [DOI] [Google Scholar]; c Marella A.; Rahmat Ali M.; Tauquir Alam M.; Saha R.; Tanwar O.; Akhter M.; Shaquiquzzaman M.; Mumtaz Alam M. Pyrazolines: A Biological Review. MiniRev. Med. Chem. 2013, 13, 921. 10.2174/1389557511313060012. [DOI] [PubMed] [Google Scholar]; d Blair L. M.; Sperry J. Natural Products Containing a Nitrogen–Nitrogen Bond. J. Nat. Prod. 2013, 76, 794. 10.1021/np400124n. [DOI] [PubMed] [Google Scholar]; e Zhang D.; Song H.; Qin Y. Total Synthesis of Indoline Alkaloids: A Cyclopropanation Strategy. Acc. Chem. Res. 2011, 44, 447. 10.1021/ar200004w. [DOI] [PubMed] [Google Scholar]
- a Harris P. A.; Faucher N.; George N.; Eidam P. M.; King B. W.; White G. V.; Anderson N. A.; Bandyopadhyay D.; Beal A. M.; Beneton V.; Berger S. B.; Campobasso N.; Campos S.; Capriotti C. A.; Cox J. A.; Daugan A.; Donche F.; Fouchet M. H.; Finger J. N.; Geddes B.; Gough P. J.; Grondin P.; Hoffman B. L.; Hoffman S. J.; Hutchinson S. E.; Jeong J. U.; Jigorel E.; Lamoureux P.; Leister L. K.; Lich J. D.; Mahajan M. K.; Meslamani J.; Mosley J. E.; Nagilla R.; Nassau P. M.; Ng S. L.; Ouellette M. T.; Pasikanti K. K.; Potvain F.; Reilly M. A.; Rivera E. J.; Sautet S.; Schaeffer M. C.; Sehon C. A.; Sun H.; Thorpe J. H.; Totoritis R. D.; Ward P.; Wellaway N.; Wisnoski D. D.; Woolven J. M.; Bertin J.; Marquis R. W. Discovery and Lead-Optimization of 4,5-Dihydropyrazoles as Mono-Kinase Selective, Orally Bioavailable and Efficacious Inhibitors of Receptor Interacting Protein 1 (RIP1) Kinase. J. Med. Chem. 2019, 62 (10), 5096. 10.1021/acs.jmedchem.9b00318. [DOI] [PubMed] [Google Scholar]; b Joshi G.; Sharma M.; Kalra S.; Gavande N. S.; Singh S.; Kumar R. Design, synthesis, biological evaluation of 3,5-diaryl-4,5-dihydro-1H-pyrazole carbaldehydes as non-purine xanthine oxidase inhibitors: Tracing the anticancer mechanism via xanthine oxidase inhibition. Bioorg. Chem. 2021, 107, 104620. 10.1016/j.bioorg.2020.104620. [DOI] [PubMed] [Google Scholar]; c Salgin-Goksen U.; Telli G.; Dedecengiz E.; Tel B. C.; Kaynak F. B.; Yelekci K.; Ucar G.; Gokhan-Kelekci N. New 2-Pyrazoline and Hydrazone Derivatives as Potent and Selective Monoamine Oxidase A Inhibitors. J. Med. Chem. 2021, 64 (4), 1989. 10.1021/acs.jmedchem.0c01504. [DOI] [PubMed] [Google Scholar]; d Bhirud J. D.; Patil R. D.; Narkhede H. P. Sulfamic acid catalyzed synthesis of new 3,5-[(sub)phenyl]-1H-pyrazole bearing N1-isonicotinoyl: And their pharmacological activity evaluation. Bioorg. Med. Chem. Lett. 2020, 30, 127558. 10.1016/j.bmcl.2020.127558. [DOI] [PubMed] [Google Scholar]; e Li C. L.; Wu S. C.; Han G. Y.; Tu J.; Dong G. Q.; Liu N.; Sheng C. Q. Targeting fungal virulence factor by small molecules: Structure-based discovery of novel secreted aspartic protease 2 (SAP2) inhibitors. Eur. J. Med. Chem. 2020, 201, 112515. 10.1016/j.ejmech.2020.112515. [DOI] [PubMed] [Google Scholar]; f Lamie P. F.; Azmey A. F. Synthesis and biological evaluation of tetrazole derivatives as TNF-α, IL-6 and COX-2 inhibitors with antimicrobial activity: Computational analysis, molecular modeling study and region-specific cyclization using 2D NMR tools. Bioorg. Chem. 2019, 92, 103301. 10.1016/j.bioorg.2019.103301. [DOI] [PubMed] [Google Scholar]; g Deng X.; Liang K.; Tong X.; Ding M.; Zhou A.; Xia C. Copper-Catalyzed Radical Cyclization To Access 3-Hydroxypyrroloindoline: Biomimetic Synthesis of Protubonine A. Org. Lett. 2014, 16, 3276. 10.1021/ol501287x. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Hu S. P.; Du S. Y.; Yang Z. G.; Ni L. F.; Chen Z. K. Synthesis of Multi-substituted Dihydropyrazoles by Copper-Mediated [4 + 1] Cycloaddition Reaction of N-Sulfonylhydrazones and Sulfoxonium Ylides. Adv. Synth. Catal. 2019, 361, 3124. 10.1002/adsc.201900212. [DOI] [Google Scholar]; b Wei Q.; Chen J. R.; Hu X. Q.; Yang X. C.; Lu B.; Xiao W. J. Photocatalytic Radical Trifluoromethylation/Cyclization Cascade: Synthesis of CF3-Containing Pyrazolines and Isoxazolines. Org. Lett. 2015, 17 (18), 4464. 10.1021/acs.orglett.5b02118. [DOI] [PubMed] [Google Scholar]; c Zhao Q. Q.; Hu X. Q.; Yang M. N.; Chen J. R.; Xiao W. J. A visible-light photocatalytic N-radical cascade of hydrazones for the synthesis of dihydropyrazole-fused benzosultams. Chem. Commun. 2016, 52, 12749. 10.1039/C6CC05897C. [DOI] [PubMed] [Google Scholar]; d Yang M. N.; Yan D. M.; Zhao Q. Q.; Chen J. R.; Xiao X. J. Synthesis of Dihydropyrazoles via Ligand-Free Pd-Catalyzed Alkene Aminoarylation of Unsaturated Hydrazones with Diaryliodonium Salts. Org. Lett. 2017, 19, 5208. 10.1021/acs.orglett.7b02480. [DOI] [PubMed] [Google Scholar]; e Hu X. Q.; Chen J. R.; Wei Q.; Liu F. L.; Deng Q. H.; Beauchemin A.; Xiao W. J. Photocatalytic Generation of N-Centered Hydrazonyl Radicals: A Strategy for Hydroamination of β,γ-Unsaturated Hydrazones. Angew.Chem. Int. Ed. 2014, 53, 12163. 10.1002/anie.201406491. [DOI] [PubMed] [Google Scholar]; f Musacchio A. J.; Nguyen L. Q.; Beard G. H.; Knowles R. R. Catalytic Olefin Hydroamination with Aminium Radical Cations: A Photoredox Method for Direct C–N Bond Formation. J. Am. Chem. Soc. 2014, 136 (35), 12217. 10.1021/ja5056774. [DOI] [PubMed] [Google Scholar]; g Lopes S. M. M.; Cardoso A. L.; Lemos A.; Melo T. M. V. D. P. Recent Advances in the Chemistry of Conjugated Nitrosoalkenes and Azoalkenes. Chem. Rev. 2018, 118 (23), 11324. 10.1021/acs.chemrev.8b00375. [DOI] [PubMed] [Google Scholar]; h Cleghorn L. A. T.; Albrecht S.; Stojanovski L.; Simeons F. R. J.; Norval S.; Kime R.; Collie I. T.; Rycker M. D.; Campbell L.; Hallyburton I.; Frearson J. A.; Wyatt P. G.; Read K. D.; Gilbert I. H. Discovery of Indoline-2-carboxamide Derivatives as a New Class of Brain-Penetrant Inhibitors of Trypanosoma brucei. J. Med. Chem. 2015, 58 (19), 7695. 10.1021/acs.jmedchem.5b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Hou J. W.; Vázquez-González M.; Fadeev M.; Liu X.; Lavi R.; Willner I. Catalyzed and Electrocatalyzed Oxidation of l-Tyrosine and l-Phenylalanine to Dopachrome by Nanozymes. Nano Lett. 2018, 18 (6), 4015. 10.1021/acs.nanolett.8b01522. [DOI] [PubMed] [Google Scholar]; j Yu J. M.; Lu G. P.; Cai C. Photocatalytic radical cyclization of α-halo hydrazones with β-ketocarbonyls: facile access to substituted dihydropyrazoles. Chem. Commun. 2017, 53, 5342. 10.1039/C7CC01470H. [DOI] [PubMed] [Google Scholar]; k Wang Z. Y.; Yang Y. Z.; Gao F.; Wang Z. Y.; Luo Q.; Fang L. Synthesis of 5-(Trifluoromethyl) pyrazolines by Formal [4 + 1]-Annulation of Fluorinated Sulfur Ylides and Azoalkenes. Org. Lett. 2018, 20, 934. 10.1021/acs.orglett.7b03811. [DOI] [PubMed] [Google Scholar]; l Attanasi O. A.; De Crescentini L.; Favi G.; Mantellini F.; Mantenuto S.; Nicolini S. Interceptive [4 + 1] Annulation of in Situ Generated 1,2-Diaza-1,3-dienes with Diazo Esters: Direct Access to Substituted Mono-, Bi-, and Tricyclic 4,5-Dihydropyrazoles. J. Org. Chem. 2014, 79 (17), 8331. 10.1021/jo5016097. [DOI] [PubMed] [Google Scholar]; m Chen B.; Chu W. D.; Liu Q. Z. Formal [4+ 1] cycloaddition of in situ generated 1, 2-diaza-1, 3-dienes with diazo esters: facile approaches to dihydropyrazoles containing a quaternary center. RSC Adv. 2019, 9, 1487. 10.1039/C8RA08909D. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Mei G. J.; Tang X. W.; Tasdan Y.; Lu Y. X. Enantioselective Dearomatization of Indoles by an Azoalkene-Enabled (3 + 2) Reaction: Access to Pyrroloindolines. Angew. Chem., Int. Ed. 2020, 59, 648. 10.1002/anie.201911686. [DOI] [PubMed] [Google Scholar]; o Mei G. J.; Zheng W. R.; Gonçalves T. P.; Tang X. W.; Huang K. W.; Lu Y. X. Catalytic Asymmetric Formal [3 + 2] Cycloaddition of Azoalkenes with 3-Vinylindoles: Synthesis of 2,3-Dihydropyrroles. iScience. 2020, 23, 100873. 10.1016/j.isci.2020.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Wei P. S.; Wang M. X.; Xu D. C.; Xie J. W. Synthesis of 2,3-Dihydrothieno(2,3-b) quinolines and Thieno(2,3-b)- quinolines via an Unexpected Domino Aza-MBH/Alkylation/Aldol Reaction. J. Org. Chem. 2016, 81 (3), 1216. 10.1021/acs.joc.5b02369. [DOI] [PubMed] [Google Scholar]; b Cheng J.; Li W. P.; Duan Y. Q.; Cheng Y. X.; Yu S. Y.; Zhu C. J. Relay Visible-Light Photoredox Catalysis: Synthesis of Pyrazole Derivatives via Formal [4 + 1] Annulation and Aromatization. Org. Lett. 2017, 19 (1), 214. 10.1021/acs.orglett.6b03497. [DOI] [PubMed] [Google Scholar]; c Li J. P.; Zhao G. F.; Wang H. X.; Xie M. S.; Qu G. R.; Guo H. M. Highly Enantioselective Synthesis of Chiral Cyclopropyl Nucleosides via Catalytic Asymmetric Intermolecular Cyclopropanation. Org. Lett. 2017, 19 (24), 6494. 10.1021/acs.orglett.7b03110. [DOI] [PubMed] [Google Scholar]; d Pelliccia S.; Alfano A. I.; Luciano P.; Novellino E.; Massarotti A.; Tron G. C.; Ravelli D.; Giustiniano M. Photocatalytic Isocyanide-Based Multicomponent Domino Cascade toward the Stereoselective Formation of minofurans. J. Org. Chem. 2020, 85 (4), 1981. 10.1021/acs.joc.9b02709. [DOI] [PubMed] [Google Scholar]; e Dong W. H.; Yuan Y.; Xie X. M.; Zhang Z. G. Visible-Light-Driven Dearomatization Reaction to-ward the Formation of Spiro [4.5] deca-1,6,9-trien-8-ones. Org. Lett. 2020, 22 (2), 528. 10.1021/acs.orglett.9b04283. [DOI] [PubMed] [Google Scholar]; f Sumino S. H.; Matsumoto F.; Iwai T.; Ito T. Methanofullerene Synthesis via Photogenerated Fullerene Radical Anion Intermediates. J. Org. Chem. 2021, 86 (12), 8500. 10.1021/acs.joc.1c00593. [DOI] [PubMed] [Google Scholar]; g Wang J.; Zhang Q. Y.; Xie M. S.; Wang D. C.; Qu G. R.; Guo H. M. Cyclization Reaction of Donor–Acceptor Oxiranes with N, N′-Disubstituted Thioureas: A Domino Process to trans-Dihydropyrimidines. Org. Lett. 2018, 20 (20), 6578. 10.1021/acs.orglett.8b02930. [DOI] [PubMed] [Google Scholar]; h Džambaski Z.; Bondžić A. M.; Triandafillidi I.; Kokotos C. G.; Bondžić B. P. Organocatalytic, Organic Oxidant Promoted, Enamine C–H Oxidation/Cyclopropanation Reaction. Adv. Synth. Catal. 2021, 363, 4002. 10.1002/adsc.202100630. [DOI] [Google Scholar]
- For selected examples, see:; a Ba D.; Chen Y. H.; Lv W. W.; Wen S.; Cheng G. L. Copper-Catalyzed Three-Component Cascade Michael Addition/Heck-Type Alkylation/Annulation: Accessing Fully Substituted 1,3-Dihydro-2H-pyrrol-2-ones. Org. Lett. 2019, 21 (21), 8603. 10.1021/acs.orglett.9b03189. [DOI] [PubMed] [Google Scholar]; b Gockel S. N.; Buchanan T. L.; Hull K. L. Cu-Catalyzed Three-Component Carboamination of Alkenes. J. Am. Chem. Soc. 2018, 140 (1), 58. 10.1021/jacs.7b10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Zhang X. W.; He Y. H.; Li J.; Wang R.; Gu L. J.; Li G. P. CO2/Photoredox-Cocatalyzed Tandem Oxidative Cyclization of α-Bromo Ketones and Amines To Construct Substituted Oxazoles. J. Org. Chem. 2019, 84 (12), 8225. 10.1021/acs.joc.9b00283. [DOI] [PubMed] [Google Scholar]; b Chatterjee T.; Cho J. Y.; Cho E. J. Synthesis of Substituted Oxazoles by Visible-Light Photocatalysis. J. Org. Chem. 2016, 81 (16), 6995. 10.1021/acs.joc.6b00989. [DOI] [PubMed] [Google Scholar]; c Choi S.; Oh H.; Sim J.; Yu E.; Shin S.; Park C. M. Metal-Free Synthesis of Indolopyrans and 2,3-Dihydrofurans Based on Tandem Oxidative Cycloaddition. Org. Lett. 2020, 22 (14), 5528. 10.1021/acs.orglett.0c01896. [DOI] [PubMed] [Google Scholar]; d Hirata G.; Shimada T.; Nishikata T. Organo-photoredox-Catalyzed Atom-Transfer Radical Substitution of Alkenes with α-Carbonyl Alkyl Halides. Org. Lett. 2020, 22 (22), 8952. 10.1021/acs.orglett.0c03359. [DOI] [PubMed] [Google Scholar]
- Zeng R.; Shan C. Y.; Liu M.; Jiang K.; Ye Y.; Liu T. Y.; Chen Y. C. [4+ 1+ 1] Annulations of α-Bromo Carbonyls and 1-Azadienes toward Fused Benzoazaheterocycles. Org. Lett. 2019, 21 (7), 2312. 10.1021/acs.orglett.9b00598. [DOI] [PubMed] [Google Scholar]
- Fang Q. Y.; Yi M. H.; Wu X. X.; Zhao L. M. Regio- and Diastereoselective Spirocyclopropanation of Benzofuran-Derived Azadienes through 1,4-Addition-Induced Dearomatization Reaction under Mild Conditions. Org. Lett. 2020, 22 (13), 5266. 10.1021/acs.orglett.0c01987. [DOI] [PubMed] [Google Scholar]
- Yang K. C.; Li J. L.; Li Q. Z.; Leng H. J.; Shen X. D.; Liu Y.; Zhu H. P.; Lai H. Y.; Zhang X.; Dai Q. S.; Liu Y.; Zeng R.; Zhang Y.. CN109956896, 2019.
- a Zhang X.; Pan Y.; Liang P.; Pang L.; Ma X.; Jiao W.; Shao H. Oxadiazepine Synthesis by Formal [4 + 3] Cycloaddition of o-Chloromethyl Arylsulfonamides with Nitrones Promoted by NaHCO3. Adv. Synth. Catal. 2018, 360, 3015. 10.1002/adsc.201800663. [DOI] [Google Scholar]; b Zhang X.; Pan Y.; Liang P.; Ma X.; Jiao W.; Shao H. An Effective Method for the Synthesis of 1,3-Dihydro-2H-indazoles via N-N Bond Formation. Adv. Synth. Catal. 2019, 361, 5552. 10.1002/adsc.201901331. [DOI] [Google Scholar]
- a Shelke A. M.; Suryavanshi G. Fluoride-Assisted Synthesis of 1,4,5,6-Tetrahydropyridazines via [4 + 2] Cyclodimerization of in Situ-Generated Azoalkenes Followed by a C–N Bond Cleavage. Org. Lett. 2016, 18, 3968. 10.1021/acs.orglett.6b01551. [DOI] [PubMed] [Google Scholar]; b Zhang Z.; Zhang L.; Chen Q.; Lu T.; Zhou Q. Synthesis of functionalized tetrahydropyridazines via catalyst-free self [4 + 2] cycloaddition of in situ generated 1,2-diaza-1,3-dienes. RSC Adv. 2016, 6, 61680. 10.1039/C6RA13985J. [DOI] [Google Scholar]
- a Aggarwal V. K.; Harvey J. N.; Robiette R. On the Importance of Leaving Group Ability in Reactions of Ammonium, Oxonium, Phosphonium, and Sulfonium Ylides. Angew. Chem., Int. Ed. 2005, 44, 5468. 10.1002/anie.200501526. [DOI] [PubMed] [Google Scholar]; b Fu Y.; Wang H. J.; Chong S. S.; Guo Q. X.; Liu L. An Extensive Ylide Thermodynamic Stability Scale Predicted by First-Principle Calculations. J. Org. Chem. 2009, 74, 810. 10.1021/jo802128w. [DOI] [PubMed] [Google Scholar]; c Zaugg H. E.; Chadde F. E.; Michaels J. R. Neighboring Group Reactions. VI. Reactions of 3-(ω-Haloalkyl)-3-phenyl-2-benzofuranones with Secondary Amines. Trapped Tetrahedral Intermediates in a Carbonyl Addition Reaction. J. Am. Chem. Soc. 1962, 84, 4567. 10.1021/ja00882a037. [DOI] [Google Scholar]
- CCDC 2013334 contains the supplementary crystallographic data for compound 3e. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- a Tanemura K.; Suzuki T.; Nishida Y.; Satsumabayashi K.; Horaguchi T. A Mild and Efficient Procedure for α-bromination of Ketones Using N-bromosuccinimide Catalysed by Ammonium Acetate. Chem. Commun. 2004, 470. 10.1039/B315340A. [DOI] [PubMed] [Google Scholar]; b Chen J. R.; Dong W. R.; Candy M.; Pan F. F.; Jorres M.; Bolm C. Enantioselective Synthesis of Dihydropyrazoles by Formal [4 + 1] Cycloaddition of in Situ-Derived Azoalkenes and Sulfur Ylides. J. Am. Chem. Soc. 2012, 134, 6924. 10.1021/ja301196x. [DOI] [PubMed] [Google Scholar]; c Hu X. Q.; Chen J. R.; Gao S.; Feng B.; Lu L. Q.; Xiao W. J. [4 + 3] Cycloaddition of in situ Generated Azoalkenes with C,N-cyclic Azomethineimines: Efficient Synthesis of Tetrazepine Derivatives. Chem. Commun. 2013, 49, 7905. 10.1039/c3cc43888k. [DOI] [PubMed] [Google Scholar]; d Wagner A. M.; knezevic C. E. K.; Wall J. L.; Sun V. L.; Buss J. A.; Allen L. T.; Wenzel A. G. A copper(II)-catalyzed, sequential Michael–aldol reaction for the preparation of 1,2-dihydroquinolines. Tetrahedron. Lett. 2012, 53, 833. 10.1016/j.tetlet.2011.12.017. [DOI] [Google Scholar]; e Yang Q. Q.; Xiao C.; Lu L. Q.; An J.; Tan F.; Li B. J.; Xiao W. J. Synthesis of Indoles through Highly Efficient Cascade Reactions of Sulfur Ylides and N-(ortho-Chloromethyl)aryl Amides. Angew. Chem., Int. Ed. 2012, 51, 9137. 10.1002/anie.201203657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


