Figure 4. Nectar bacteria exert negative priority effects against nectar yeast, potentially due to reduction in nectar pH.
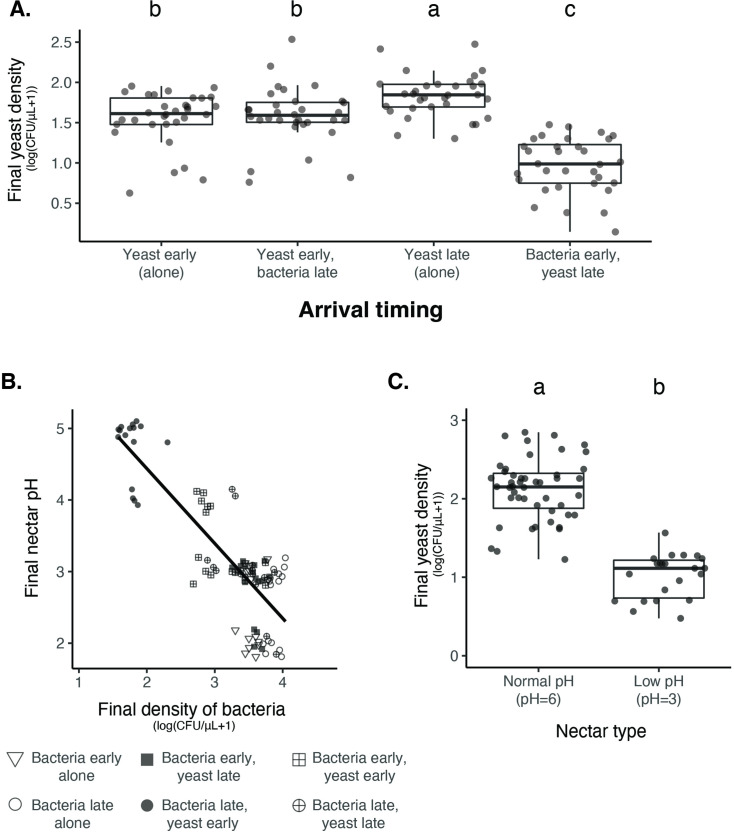
(A) Metschnikowia reukaufii (strain MR1) yeast population density after five days of growth with alternating arrival order with Acinetobacter nectaris bacteria or growth alone with inoculation on either the first or third day (arriving early or late) of the experiment (n=128). (B) Final pH of nectar after 5 days of bacterial growth; higher densities of bacteria are associated with lower pH. The shape of each point represents the treatments represented in panel A. Points are jittered on the y-axis (n=96). (C) Low pH (pH = 3) nectar depresses yeast growth when grown in low-density monoculture (n=72). In box plots (panels A and C), treatments that share the same letter placed above their boxes were statistically indistinguishable from one another.
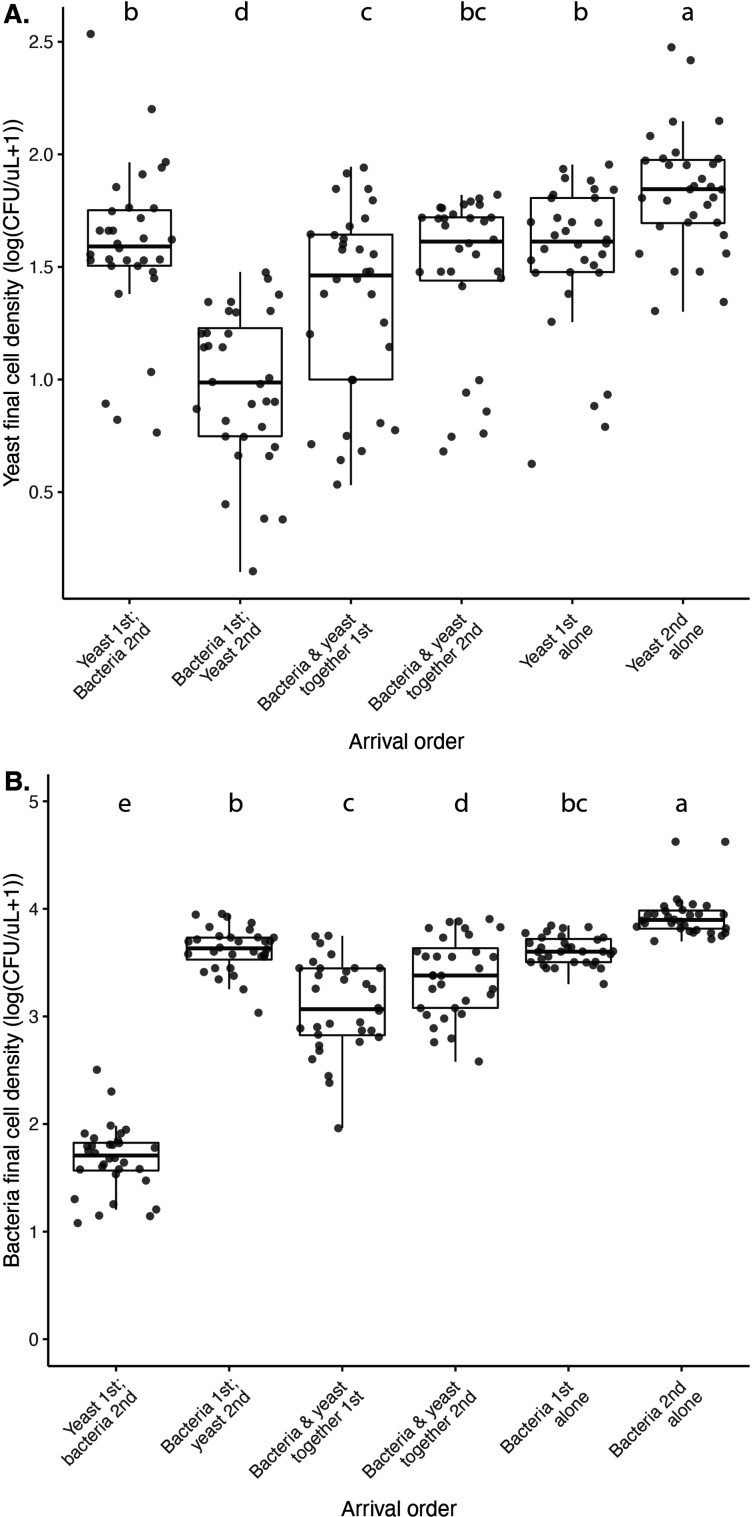
Figure 4—figure supplement 1. M. reukaufii yeast and A. nectaris bacteria exhibit negative priority effects against each other, as evidenced by growth in microcosm experiments where arrival order is altered.
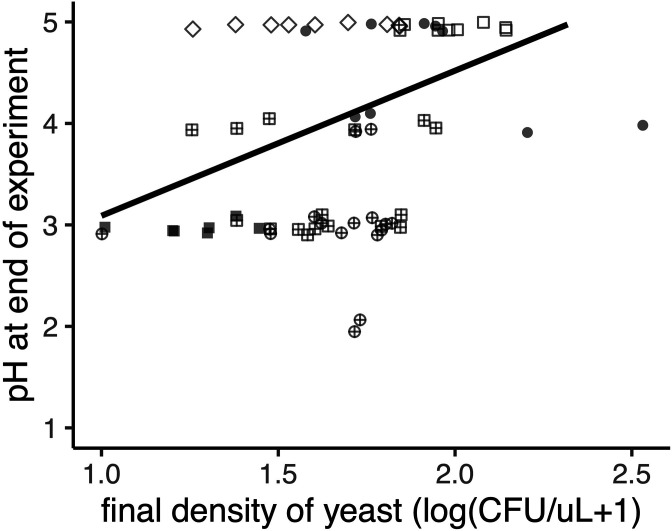
Figure 4—figure supplement 2. Yeast increases nectar pH (p<0.05, Spearman rank correlation).
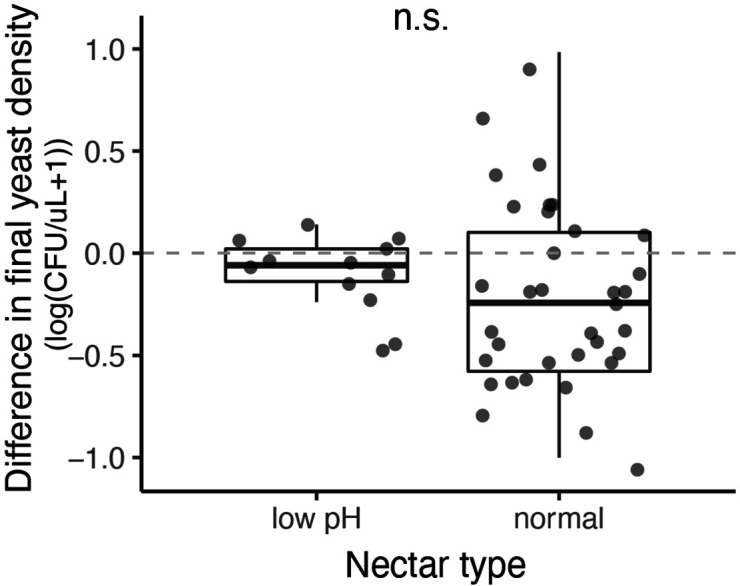
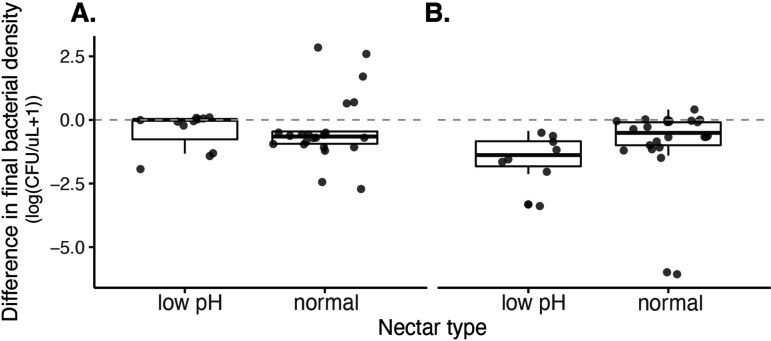
Figure 4—figure supplement 3. We found no effect of nectar type (pH=3, pH=6) on the growth of M. reukaufii, when grown in monoculture at a high density (approximately 10,000 cells/µL).