FIG 1.
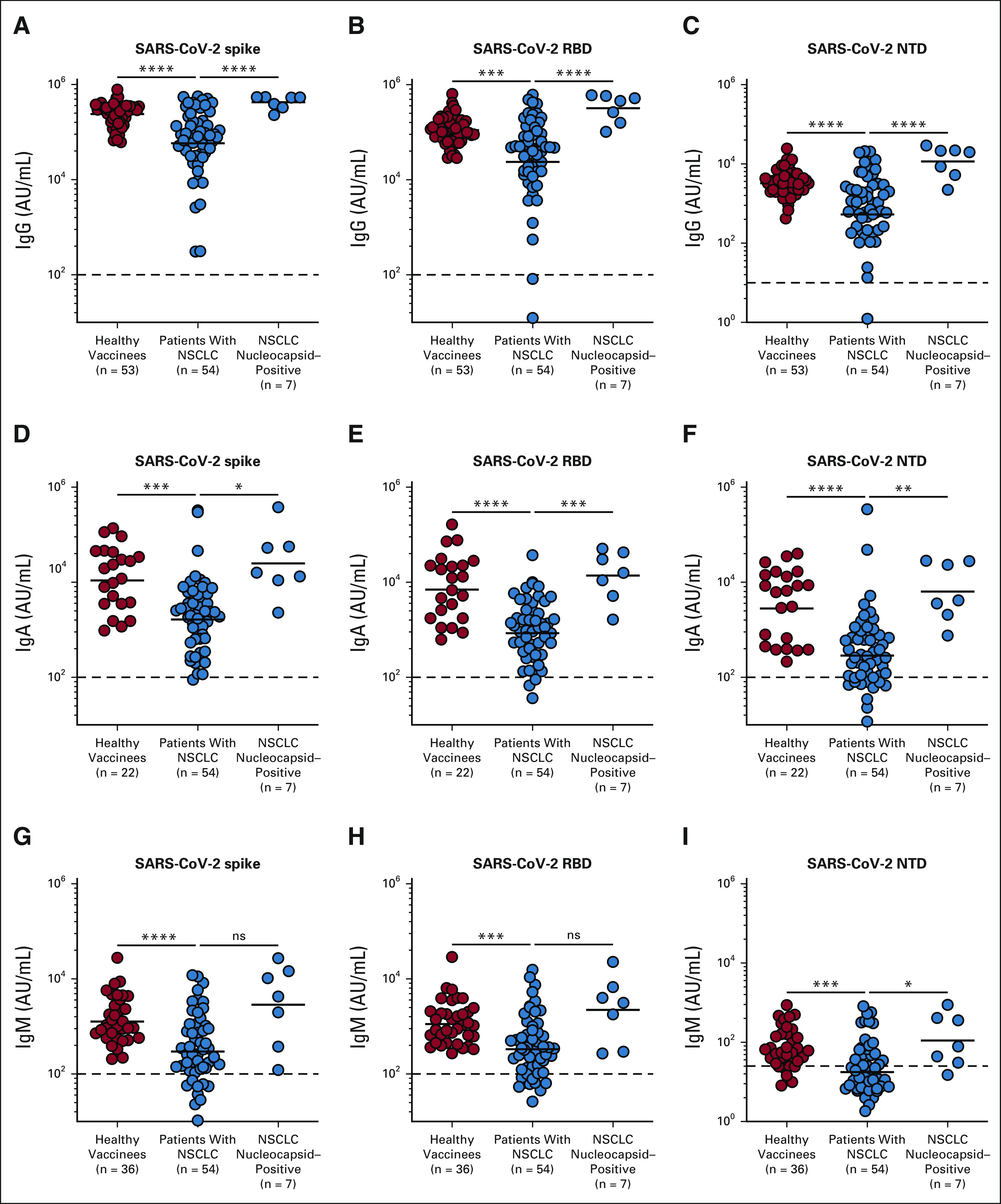
Antibody response to SARS-CoV-2 mRNA vaccine in patients with NSCLC. Binding antibody titers to spike, RBD, and NTD antigens were measured by MSD assay 1 month after the two-dose primary vaccination. Spike-, RBD-, and NTD-specific antibody titers in plasma from healthy vaccinees, patients with NSCLC, and patients with NSCLC with prior exposure to SARS-CoV-2 infection are shown for (A-C) IgG, (D-F) IgA, and (G-I) IgM, respectively. Prepandemic plasma samples from healthy individuals were used to set the detection limit for SARS-CoV-2–specific antibody titers. The figures show the mean and SEM. *P ≤ .05, **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; MSD, Meso Scale Discovery; ns, not significant; NSCLC, non–small-cell lung cancer; NTD, N-terminal domain; RBD, receptor-binding domain.
