Abstract
Repetitive bouts of binge drinking can lead to neuroplastic events that alter ethanol’s pharmacologic effects of and perpetuate excessive consumption. The corticotropin-releasing factor (CRF) system is an example of ethanol-induced neuroadaptations that drive excessive ethanol consumption. Our laboratory has previously shown that CRF antagonist, when infused into the central amygdala (CeA), reduces binge-like ethanol consumption. The current study extends this research by assessing the effects of silencing CRF-producing neurons in CeA on binge-like ethanol drinking stemming from “Drinking in the Dark” (DID) procedures. CRF-ires-Cre mice underwent surgery to infuse Gi/o-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADD) virus or a control virus into either the CeA or basolateral amygdala (BLA). Gi/o-DREADD-induced CRF neuronal inhibition in the CeA resulted in a 33% decrease in binge-like ethanol consumption. However, no effect on ethanol consumption was seen after DREADD manipulation in the BLA. Moreover, CeA CRF neuronal inhibition had no effect on sucrose consumption. The effects of silencing CRF neurons in the CeA on ethanol consumption are not secondary to changes in motor function or anxiety-like behaviors as assessed in the open field test. Finally, the DREADD construct’s functional ability to inhibit CRF neuronal activity was demonstrated by reduced ethanol-induced c-Fos following DREADD activation. Together, these data suggest that the CRF neurons in the CeA play an important role in binge ethanol consumption and that inhibition of the CRF signaling pathway remains a viable target for manipulating binge-like ethanol consumption.
Keywords: Alcohol Use Disorder, Corticotropin-releasing factor, Binge Drinking, Central Amygdala, chemogenetics
1. Introduction
Excessive alcohol (ethanol) use, and in particular binge drinking, is burdensome to the US economy (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011) and promotes poor decision making (Goudriaan, Grekin, & Sher, 2007). to the consequences from binge drinking can range from vehicular accidents to increased incidences of interpersonal violence (Bouchery et al., 2011; Sloan, Eldred, & Davis, 2014; Stahre, Brewer, Fonseca, & Naimi, 2009). Binge drinking is defined as a pattern of ethanol consumption that results in blood ethanol concentrations (BECs) greater than 80mg/dL (NIAAA, 2004). This problematic pattern of drinking, not only causes economic and sociological issues, but is also hypothesized as being a risk factor for developing alcohol use disorders (AUDs).
One hypothesis for AUD development is that neurobiological maladaptations occur in response to binge-like ethanol drinking (Sprow & Thiele, 2012; Thiele & Navarro, 2014). While there are many neuroadaptations that have been explored for their contributions to AUDs including neuroimmune function (Crews et al., 2015), the balance between GABA-ergic and glutamatergic signaling (Marisa Roberto & Varodayan, 2017), and epigenetic mechanisms (Palmisano & Pandey, 2017), to name just a few, this study focuses on ethanol-induced neuroadaptations in the corticotropin-releasing factor (CRF) system which has been shown to play a critical role in AUDs (Curley, Webb, Sheffler, & Haass-Koffler, 2021). CRF is a neuropeptide that consist of just 41 amino acids but is heavily involved with behavioral and physiological changes associated with stress (Deussing & Chen, 2018; Hauger, Risbrough, Brauns, & Dautzenberg, 2006). Both clinical and preclinical evidence indicates there is an association between stress, particularly the CRF system, and ethanol dependence (Becker, 2017; Breese, Sinha, & Heilig, 2011; M. Roberto, Kirson, & Khom, 2021). Given the pivotal role that CRF plays in stress responses and that stress has been linked to excessive alcohol consumption, many studies of alcohol dependence have linked CRF signaling directly with ethanol consumption. For example, CRF inhibition in animals that were made dependent via ethanol vapor exposure reduced ethanol consumption in subsequent operant tasks (M. Roberto et al., 2010). However, because alcohol has anxiolytic effects in non-dependent individuals in the clinic, the CRF system in the amygdala may also underlie binge drinking mechanisms(Gilman, Ramchandani, Davis, Bjork, & Hommer, 2008). Our lab has previously shown that CRF antagonist in the central amygdala (CeA), but not the adjacent basolateral amygdala (BLA), reduces binge-like ethanol consumption (Lowery-Gionta et al., 2012). While these observations show the CRF receptor signaling in the CeA modulates binge-like ethanol drinking, the source of CRF that modulates excessive drinking cannot be identified by these pharmacological approaches. Thus, it is unclear if CRF released by local neurons within the CeA directly modulate binge drinking or whether the CRF input into the CeA from regions like the BNST or PVH are necessary in these behavioral responses.
Utilizing designer receptors exclusively activated by designer drugs (DREADDs) allows investigators to manipulate discrete populations of neurons with anatomical precision while reducing off-target effects compared to other psychopharmacological methods that often require cannulation and/or non-selective drugs (Lee, Giguere, & Roth, 2014; Smith, Bucci, Luikart, & Mahler, 2016). The studies herein elucidate the role of CRF neurons in male mice using the “Drinking in the Dark” (DID) paradigm. The DID paradigm is a rodent model of binge-like drinking uniquely suited to model the window of heavy ethanol consumption prior to dependence (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Thiele & Navarro, 2014). Because the rodents self-administer ethanol within the DID paradigm, it is possible to test whether chemogenetic manipulations impact binge drinking. This study tests the hypothesis that the local CRF-producing neurons in the CeA can modulate ethanol consumption by measuring alcohol consumption after CRF-neuronal inhibition.
2. Methods
2.1. Animals
CRF-ires-Cre (CRF-Cre) mice were bred in-house backcrossed on a C57BL/6J background in an AALAC accredited vivarium maintained at 22°C with a reversed 12:12 hour light:dark cycle (lights off at 8:30am; lights on at 8:30pm). CRF-Cre mice are positive for the expression of Cre under the CRF promoter as determined by standard PCR genotyping protocols (Pleil et al., 2015; Rinker et al., 2017). During experimental procedures, male mice were individually housed and given ad libitum access to either Prolab® RMH 3000 (Purina LabDiet®; St. Louis, MO) (Marshall et al., 2015). Female mice were excluded because the impact of ethanol on CRF and CRF receptors has not been characterized in our foundational studies (Lowery-Gionta et al., 2012). Moreover, the estrous cycle can influence behaviors that are mediated by CRF signaling (Wiersielis et al., 2016). During surgical recovery and behavioral studies, mice were individually housed without any environmental enrichment. Single-housing was necessary to get accurate measurements of individual drinking as well as because both social dynamics (Fulenwider, Robins, Caruso, & Ryabinin, 2021) and environmental enrichment can impact ethanol consumption (Holgate, Garcia, Chatterjee, & Bartlett, 2017). The procedures used in this study were all approved by the University of North Carolina Institutional Animal Care and Use Committee and followed the Guidelines for the Care and Use of Laboratory Animals (NRC, 2011). Figure 1 illustrates the timeline of animal procedures performed.
Figure 1.
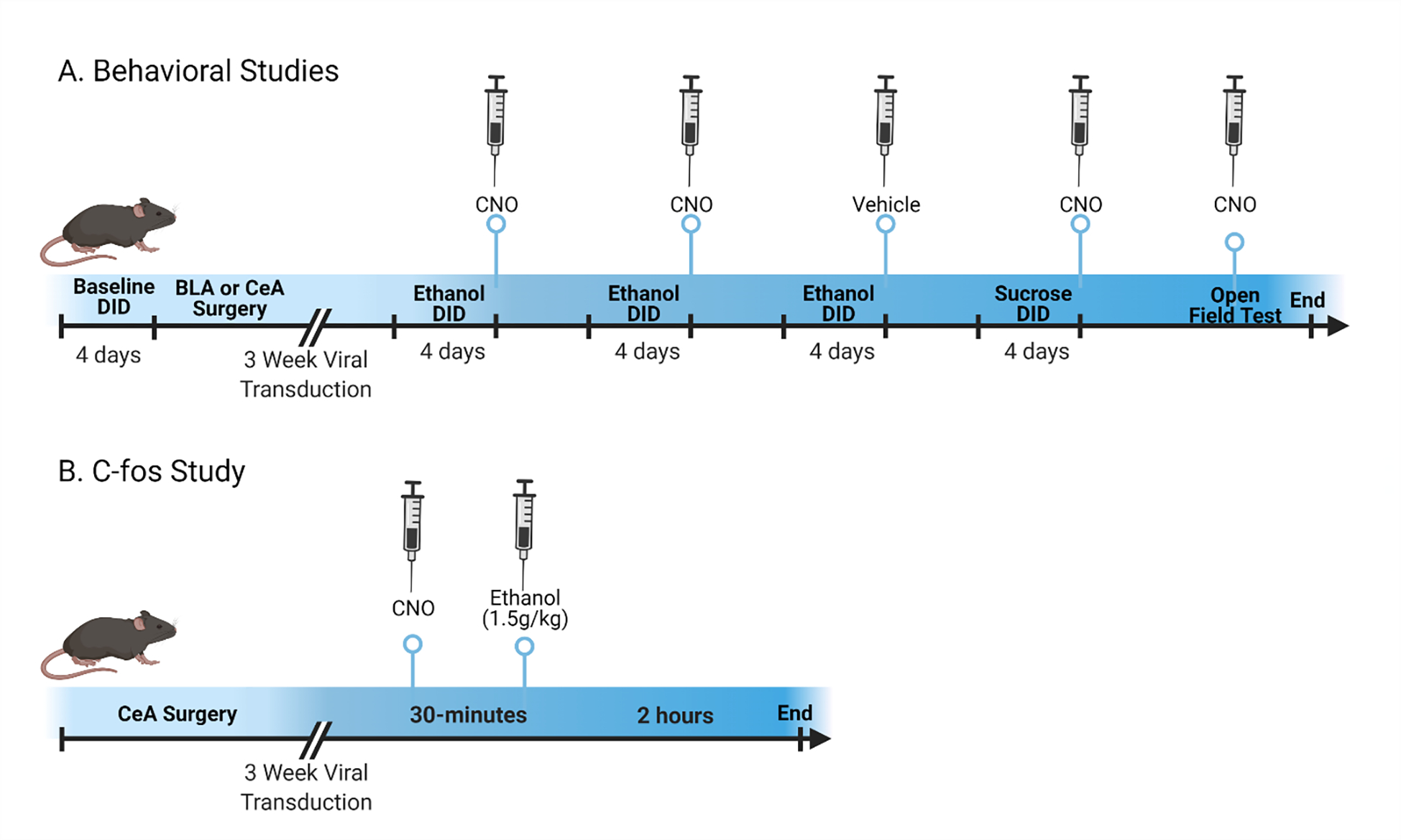
Mice used in behavioral studies (A) underwent surgery in the BLA or CeA receiving either Gi/o-DREADD or control virus after a baseline was established in the DID with ethanol. After a recovery period, the impact of Gi/o-DREADD activation on ethanol consumption was determined in 2 cycles of DID with CNO. To ensure the CNO did not have an independent effect, a third DID cycle was conducted using vehicle on the test day. As a follow-up, mice that were used in the CeA studies also underwent 1 cycle of sucrose DID consumption with CNO. Finally, the same mice were tested in an OFT with CNO to compare the Gi/o-DREADD vs control viral impact on locomotor activity and anxiety after CNO injection. In a separate cohort for the c-Fos studies (B), viral constructs were injected into the CeA and activated with CNO. Ethanol was administered ip before animals were euthanized.
2.2. DREADD Stereotactic Surgery
Mice received an anesthetic dose (1.5mL/kg) of a ketamine (100mg/kg) and xylazine (10mg/kg) cocktail prior to surgery. Mice were stereotaxically injected with either a Cre-dependent control virus (AAV8-hSyn-DIO-mCherry; UNC Vector Core, Chapel Hill, NC) ) or a Cre-dependent Gi/o-coupled DREADD vector (AAV8-hSyn-DIO-hM4d-mCherry; UNC Vector Core) into the BLA (AP:−1.22, ML:±3.01, DV: −4.70) or CeA (AP: −1.05 ML:+/−2.5 DV:−4.64) (Paxinos, 2009). The virus was delivered using a Hamilton syringe to inject a total of 0.3 μl bilaterally at a rate of 0.1 μl per minute. After each injection, the syringe remained in place for ten minutes for proper virus diffusion. Following surgery, mice were given at least three weeks of rest prior to any other procedures to ensure DREADD protein expression, translocation, and incorporation into CRF+ cells. Clozapine-N-oxide (CNO; 0.6mg/mL; 1%DMSO in 0.9% saline) was administered intraperitoneally (3mg/kg) to activate the DREADD receptor. CNO is an agonist specific to DREADD receptors that is otherwise inert (Lee et al., 2014).
2.2. “Drinking in the Dark” & Consumption Study
Binge drinking was modeled using a well-established 4 day DID paradigm exactly as previously described (Marshall et al., 2015; Rhodes et al., 2005). Briefly, homecage water bottles were removed and animals were given access to sipper tubes of a 20% (v/v) ethanol solution or 3% (w/v) sucrose three hours into the dark cycle. Sipper tubes were available for a period of two hours on days 1–3, but on the fourth day, or test day, the ethanol or sucrose was available for four hours. This four-day procedure followed by three days of abstinence constitutes 1 cycle. Prior to surgery, mice used in the consumption study were given 1 cycle with ethanol to ensure that all groups were equally matched and consumed binge-like levels of ethanol. Mice transfected with either control or Gi/o virus underwent two consecutive cycles of ethanol DID. On the first two test days, mice were given CNO (3mg/kg) approximately 30 mins before the DID procedure to determine the effects of CRF-neuronal inhibition within the CeA (n=22) on ethanol consumption. The BLA (n=16) was used as an anatomical control as mice express CRF receptors but not CRF producing neurons in the BLA (Kono et al., 2017; Wang et al., 2011). In mice with CeA DREADD manipulations, animals then underwent a third cycle where vehicle (1%DMSO in 0.9% saline) was administered (5mL/kg) instead of CNO to ensure the results were not an artifact of the virus independent of DREADD activation. Tail blood samples (≈60μL) were taken immediately following each test day to determine BECs. Serum was obtained by centrifugation and run in duplicate on the AM1 Alcohol Analyzer (Analox, London, UK) and averaged for BEC determination (mg/dL). Finally, mice with control or Gi/o virus within the CeA underwent two cycles of sucrose DID and were given CNO to determine if CRF-neuronal inhibition effects were specific to ethanol or generalized to other caloric rewarding solutions.
2.3. Open Field Tests & Elevated Plus Maze
An open-field test (OFT) was used to determine if CRF-neuronal inhibition within the CeA had an effect on locomotor activity or anxiety-like behaviors (Cox et al., 2013; Prut & Belzung, 2003). Similar to previous reports (Fee et al., 2004), mice received CNO prior to being placed in chambers and VersaMax® software program (AccuScan Instruments, Inc., Columbus, OH) tracked their movement over a two hour period with five minute output-bins using VersaDat® Version 4.00 (AccuScan Instruments, Inc.). Total distance traveled was measured as a correlate of locomotor activity, and center distance and time were recorded to determine if CeA CRF-neuronal inhibition altered anxiety-like behavior (Prut & Belzung, 2003).
2.4. CRF-Cre C-Fos Study
Mice that were surgically infused with the Gi/o DREADD or control virus, as previously described herein, were used to assess the ability of the Gi/o DREADD construct to inhibit CRF neuronal signaling within the CeA. Mice were given CNO thirty minutes prior to administration of 1.5g/kg ethanol ip (13% v/v in 0.9% saline). This dose of ethanol has previously been indicated to increase c-Fos within the amygdala and simulates BECs comparable to those achieved within the DID paradigm (Marshall et al., 2015; McBride, 2002; Robinson et al., 2020). Two hours post ethanol injections, mice were euthanized via perfusion.
2.5. Tissue Procurement
Following all experiments, mice were overdosed with a 0.15mL intraperitoneal (ip) injection of an anesthetic cocktail of ketamine and xylazine (66.67mg/mL; 6.67mg/mL;0.9% saline). Transcardial perfusion with 0.1M phosphate buffer saline (PBS; pH=7.4) and 4% paraformaldehyde in PBS (pH=7.4) fixated the brains prior to extraction. For the mice used in the consumption and behavioral tasks, brains were then postfixed for 24h; however, brains taken for c-Fos were only post-fixed for 2h. Regardless of post-fixation time, 40 μm sections were obtained using a vibratome (Leica VT1000S; Wetzlar, Germany) and stored in a 1:4 series for further processing.
2.6. Immunohistochemistry
Every fourth section collected from c-Fos experimental brains was used for c-Fos immunoreactivity detection similar to previous description of immunohistochemical procedures (Robinson et al., 2020). Briefly, sections were rinsed in 0.1M PBS before submersion in goat serum (PBS/0.1% triton-X/3% serum; Vector Laboratories; Burlingame, CA) for thirty minutes to reduce background staining. Sections were then incubated in rabbit c-Fos primary antibody (1:2500; Santa Cruz Biotechnology; Santa Cruz, CA) for 48 hours at 4°C. After serial washes in goat block to remove excess primary antibody, the tissue was then incubated in goat anti-rabbit secondary (1:200), an avidin-biotin-peroxidase complex for amplification (ABC elite kit, Vector Labs), and finally, the chromagen, 3,3’-diaminobenzidine tetrahydrochloride (Polysciences; Warrington, PA). Sections were mounted onto glass slides and coverslipped using SHUR/Mount™ (Triangle Biomedical Sciences; Durham, NC). Profile counts were used to determine the impact of the Gi/o-DREADD construct on neuronal activation (c-Fos+ cells/section).
Sections from brains collected from consumption studies were used to verify DREADD virus expression occurred solely in CRF expressing neurons. To this end, slices were blocked in 10% donkey serum and 0.1% Triton X-100 in PBS for 1 h. Sections were then transferred to fresh PBS containing primary antibody for 72 h at 5°C. CRF expression was detected using primary rabbit anti-CRF (ab8901; 1:250; Abcam, Cambridge, MA). Sections were then rinsed two times in PBS to remove excess primary antibody. The sections were then incubated in donkey anti-rabbit IgG H&L (Alexa Fluor 488) secondary (ab150073; Abcam, Cambridge, MA). Following two final rinses in PBS, all sections were mounted on glass slides, air-dried overnight, and coverslipped for viewing. Digital images of each candidate brain region were obtained on a Leica DM6000B imaging microscope. Correct DREADD placement was verified using anatomic landmarks with the aid of a mouse stereotaxic atlas.
2.7. Statistical Analysis
Prism Version 7.01 (GraphPad Software, Inc. La Jolla, Ca) was used to analyze and graph all data reported herein. Two-way analyses of variance (ANOVAs) were used to determine the effect of CRF neuronal inhibition on consumption, BECs, and open-field test parameters. Bonferroni corrected t-tests were only conducted if a significant interaction or main effect of treatment was observed. c-Fos expression, consumption after saline administration, and pre-surgery consumption were analyzed with unpaired, two-tailed t-tests. Each subregion of the amygdala was independently analyzed. All data are reported as the mean ± standard error of the mean and considered significant if p<0.05, two-tailed. The datasets for these studies are available upon request.
3. Results
3.1. DREADD manipulation in the CeA, but not BLA, Reduces Binge-Like Ethanol Consumption
Prior to surgery, similar ethanol consumption was observed for mice used for both the CeA (t(20)=1.05, p=0.31) and BLA (t(14)=0.48, p=0.63) experiments between animals receiving the control (CeA μ= 4.89± 0.35 ; BLA μ= 4.56± 0.65 g/kg) and Gi/o DREADD (CeA μ= 4.33± 0.40 ; BLA μ= 4.93± 0.32 g/kg) according to t-tests. A two-way repeated measures (RM) ANOVA (viral condition × time) indicated that viral condition in the CeA [F(1,20)=14.87, p=0.001] but not time [F(1,20)=1.37, p=0.26] or an interaction [F(1,20)=0.83, p=0.37] significantly impacted ethanol consumption (Figure 2A). CNO-induced neuronal inhibition in the CeA through the Gi/o construct reduced ethanol consumption by approximately 33%. Consumption corresponded with BECs as there was a main effect of viral condition [F(1,20)=5.17, p=0.03] but not time [F(1,20)=0.13, p=0.72] or an interaction ([F(1,20)=0.92, p=0.35]; Figure 2B). To ensure that the viral construct alone did not alter ethanol consumption, all mice were given vehicle during the third DID ethanol cycle. A t-test indicated, in the absence of CNO, that viral construct had no effect on ethanol consumption (t(20)=0.48, p=0.63; Figure 2C) or blood ethanol concentration (t(18)=0.11, p=0.91; Figure 2D). The effect of neuronal inhibition was specific to ethanol as no effect of viral treatment with CNO was observed on sucrose consumption (t(20)=0.10, p=0.92; Figure 2E). Moreover, a two-way RM ANOVA (viral condition × time) indicated no main effect of viral condition in the BLA [F(1,14)=1.70, p=0.21] or time [F(1,14)=2.60, p=0.13] and no interaction between time and viral condition ([F(1,14)=1.52, p=0.24]; Figure 3A) on ethanol consumption or blood ethanol concentrations (viral condition: [F(1,10)=0.58, p=0.80] time: [F(1,10)=1.08, p=0.32] interaction: [F(1,10)=0.08, p=0.78]). One cohort’s BECs from test 2 were lost due to mechanical malfunctions of the centrifuge; however, given the lack of effect on consumption, it is unlikely that it would alter the analyses (Figure 3B).
Figure 2.
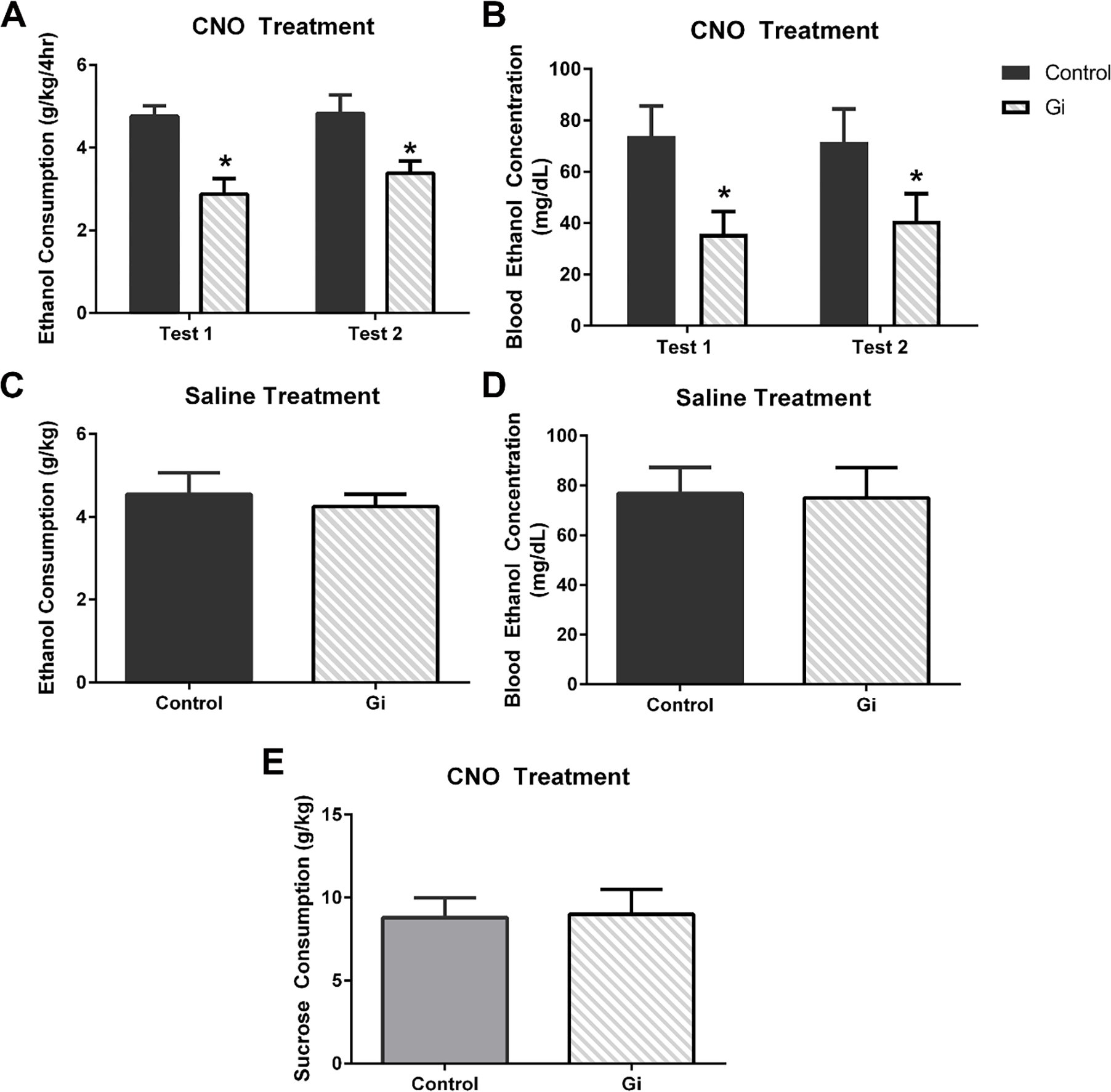
Silencing of CRF neurons in the CeA reduced binge-like ethanol consumption (A) and BECs (B) in the Gi/o-DREADD mice (n=11) compared with mice with control virus (n=10). In the absence of CNO, however, viral condition did not alter ethanol consumption (C) and therefore had no effect on BECs (D). Finally, no effect of silencing CRF neurons was observed on sucrose consumption (E) compared with the control virus condition group. (*p<0.05 compared to control virus group). All data are presented as mean ± SEM.
Figure 3.
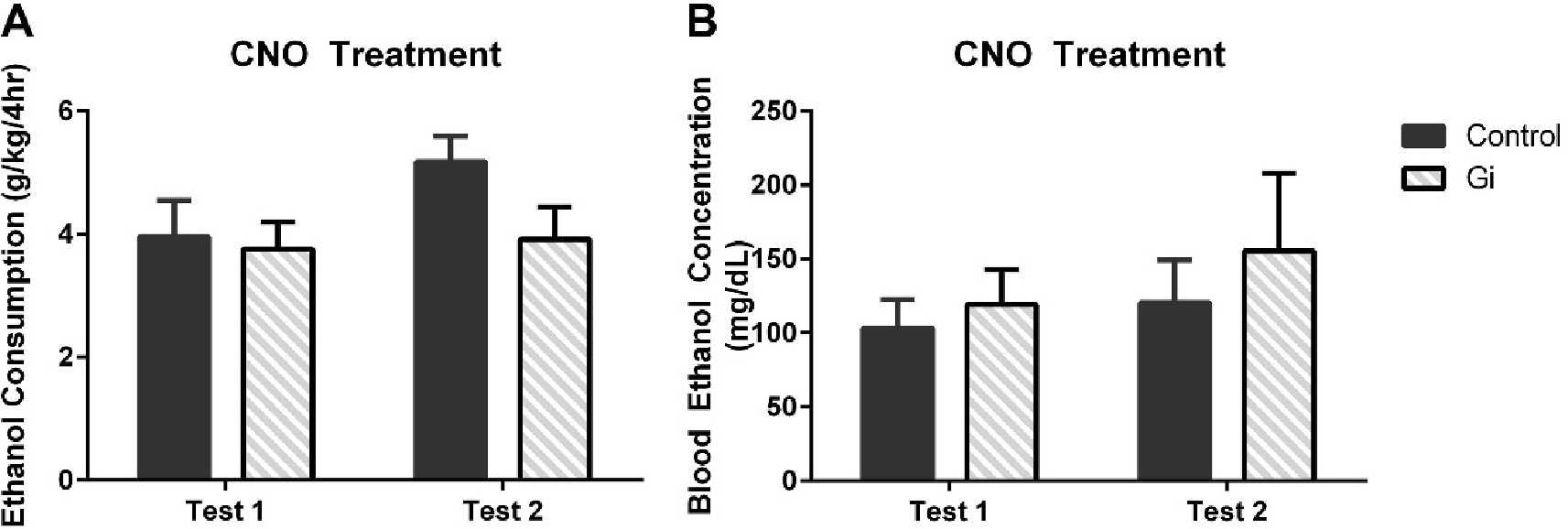
Gi/o-DREADD viral administration (n=8) within the BLA had no effect on either ethanol consumption (A) or BECs (B) compared with the control group (n=7). All data are presented as mean ± SEM.
3.2. Silencing of CRF Neurons in the CEA has No Effect on Open-Field Test Measures
Following all consummatory experiments, mice surgically delivered DREADD or control virus within the CEA were subjected to an open-field test to determine the effects of CRF-neuronal inhibition on locomotor activity and anxiety-like measures. There was no difference in locomotor activity between mice that received Gi/o or control virus within the CeA and CNO injection on the test day. Two-way repeated measures ANOVAs only indicated a significant main effect of time [F(47,893)= 5.80, p<0.0001; F(47,893)= 6.37, p<0.0001] but not viral condition [F(1,19)= 0.79, p=0.39; F(1,19)= 2.31, p=0.15] or any interaction [F(47,893)=0.56, p=0.99; F(47,893)= 0.47, p=0.99] on total distance traveled or distance traveled in center, respectively (Table 1). Moreover, no significant main effect of treatment, time, or interactions were found analyzing total time spent in the center [F(1,19)=1.35, p=0.26; F(47,893)=1.01, p=0.45; F(47,893)=0.43, p=0.99] (Table 1).
Table 1.
Measurements of Anxiety-Like Behaviors and Locomotion in the OFT
| Treatment | Distance Traveled (cm) | Center Distance (cm) | Center Time (min) |
|---|---|---|---|
| Control (n=10) | 21298±2401 | 7862±934 | 60.3±4.1 |
| Gi/o (n=11) | 18292±2380 | 5955±843 | 50.3±7.2 |
3.3. Gi/o DREADD Activation Reduces Ethanol-Induced c-Fos+ Cells in the CeA
In a separate cohort of animals, the ability of Gi/o -DREADDs to inhibit neuronal activation was validated using ethanol-induced c-Fos upregulation. Photomicrographs reveal a reduction in c-Fos+ cells in the Gi cohort (Figure 4B) compared with the controls (Figure 4A). A t-test confirmed this significant effect of Gi-DREADD induced CRF neuronal inhibition on c-Fos+ cells within the CeA [(t(19)=7.13, p<0.0001); (Figure 4C)]. Qualitative analyses suggest that our viral manipulations were centered in the CeA and were found in CRF+ cells.
Figure 4.
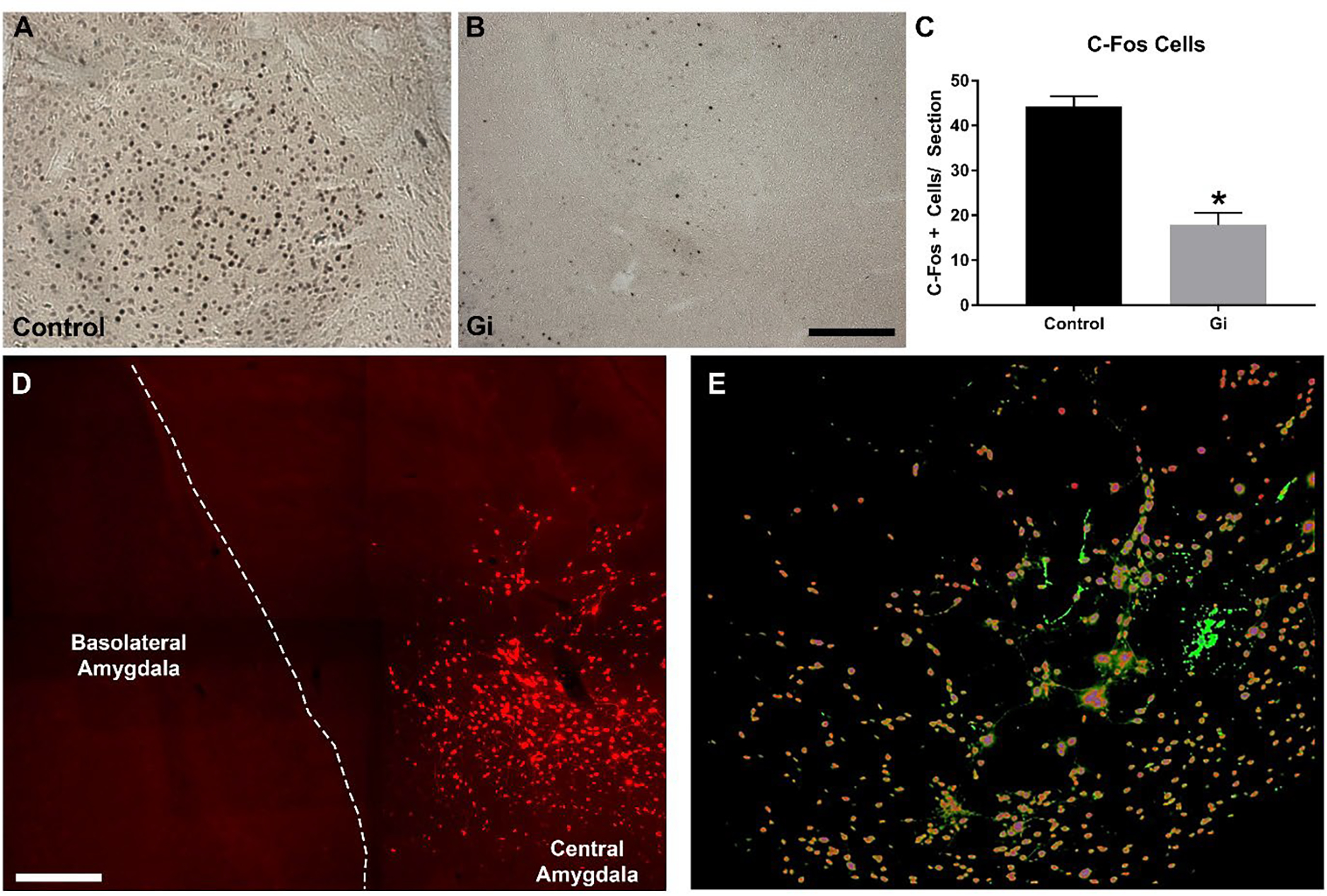
Photomicrographs of mice with either control (A; n=9) or Gi/o (B; n=12) DREADD show a depression of ethanol-induced c-Fos IR in mice with activated Gi/o DREADD. Direct comparisons show that CRF neuronal inhibition within CeA reduced c-Fos expression in Gi/o animals compared with controls (C). Panel D is a multi-panel image capturing the amygdala indicating that DREADD expression was confined to the CeA. Panel E is a colabel showing the overlap of CRF+ (green) and DREADD+ cells (red) within the CeA.
4. Discussion
The current study builds upon our previous findings that repeated binge-like consumption increases CRF expression and that pharmacological manipulation of the CRF system modulates ethanol consumption (Lowery-Gionta et al., 2012; Sparta et al., 2004); however, these experiments specifically pinpoint CRF-neuronal signaling within the central amygdala as a critical player in binge-like ethanol consumption. The results showed that Cre-induced DREADD inhibition of CRF neurons in the CeA reduced binge-like ethanol consumption, but DREADD virus transduction in the BLA, a region devoid of CRF production, failed to alter binge-like ethanol intake. This latter observation confirms that off-target effects of virus bleed from the CeA to adjacent brain regions are unlikely to account for DREADD-induced reductions of binge-like ethanol intake. There was no significant difference in ethanol consumption in mice treated with vehicle, indicating that the virus alone did not have an effect outside of CNO administration. Additionally, there was no significant effect of Gi/o-DREADD activation on sucrose consumption, suggesting that the effect of CRF receptor antagonism in the CeA is specific to ethanol consumption and does not affect general rewarding solutions. Finally, c-Fos cell counts confirmed that Gi/o -DREADD activation inhibited neuronal activation and colabeling procedures showed that these DREADDs were specifically in CRF+ cells within the CeA.
Our initial studies into the impact of the CRF-system on binge-like consumption began by showing that peripheral administration of CRF-1 receptor antagonist reduced excessive and stress-induced ethanol consumption (Lowery, Sparrow, Breese, Knapp, & Thiele, 2008; Sparta et al., 2004). It was then established that this effect was mediated by the central nervous system (Lowery et al., 2010), and more specifically that it was the CRF receptors in the CeA that were responsible for the reduced consumption (Lowery-Gionta et al., 2012). In fact, others have shown that manipulating CRF-1 receptors ameliorates ethanol’s impact on GABAergic neurotransmission onto the CeA (Nie et al., 2009). However, the CRF system within the CeA is not autonomous, especially as it relates to AUDs (Gilpin, Herman, & Roberto, 2015; M. Roberto, Gilpin, & Siggins, 2012). Sources of CRF can come from external projections into the CeA (Treweek, Jaferi, Colago, Zhou, & Pickel, 2009; Uryu, Okumura, Shibasaki, & Sakanaka, 1992), and CRF-1 receptors are found both pre- and post-synaptically within the CeA (Jaferi & Pickel, 2009). Together, these facts suggest that the effects of CRF-1 receptor antagonism on ethanol consumption, previously observed in this model, may be mediated by projections onto the CeA which either release CRF or express CRF receptors (Lowery-Gionta et al., 2012). However, by utilizing DREADD technology, the current data set specifically delineates CRF producing neurons within the CeA as an important mediator of binge-like ethanol consumption (Lowery-Gionta et al., 2012; Zhou, Colombo, Gessa, & Kreek, 2013). Our observations are consistent with a report that showed that optogenetic silencing of CRF+ neurons in the CeA blunted dependence-induced ethanol intake in rats (de Guglielmo et al., 2019), but contrasts with a recent observation that failed to find altered ethanol intake following silencing of CRF neurons in the CeA of mice (Kreifeldt et al., 2022). The latter report did not employ DID procedures, thus experimental paradigm differences is one factor that may account for the contrasting results between studies.
CRF-producing neurons of the CeA project to various regions including the hypothalamus (Krettek & Price, 1978) and various regions of the brain stem (Pomrenze et al., 2015). Our previous studies highlight the importance of CRF-receptor inhibition in the bed nucleus of the stria terminalis (BNST) and ventral tegmental area (VTA) (Pleil et al., 2015; Rinker et al., 2017), and both the BNST and the VTA have CRF neuronal projections from the CeA (Pomrenze et al., 2015), suggesting that these may be the circuitry underlying the reduction in consumption. Recent work has also shown that Gq-DREADD activation of the CeA CRF projections to the nucleus accumbens core (NAc) is sufficient to decrease binge ethanol intake (Borrego et al., 2022). Given gross Gi/o-DREADD mediated CeA CRF inhibition decreased drinking in our studies, these findings together underscore the critical importance of evaluating the projection specific role of CeA CRF signaling in binge intake. CRF is also co-expressed in GABAergic interneurons within the CeA which may underlie the ability of CRF-neuronal silencing to reduce binge-like consumption (Day, Curran, Watson, & Akil, 1999; Nie et al., 2009; M. Roberto et al., 2010), especially given our previous finding that CRF-1 receptor antagonism within the CeA reduced consumption (Lowery et al., 2010). Interestingly, we recently reported that chemogenetic silencing of GABAergic and NPY Y1 receptor expressing neurons arising from the CeA and innervating the lateral habenula (LHb) significantly blunted binge-like ethanol intake in male and female mice (Companion, Gonzalez, Robinson, Herman, & Thiele, in press). As CRF is co-expressed with GABA in the CeA, it is possible that blunted binge-like ethanol intake stemming from silencing CRF neurons of the CeA in the present study was modulated by reduced GABA/CRF signaling in the LHb, Although our experiments did not directly ascertain which projections are most important, the fact that CRF-neuronal inhibition within the CeA reduces ethanol consumption further supports the hypothesis that the CeA is a central-hub mediating ethanol consumption through CRF-producing neurons (Gilpin et al., 2015). The scope of this study was limited to understanding the role of CRF producing neurons but future studies should investigate the specific projections as well as co-expression patterns in the CRF-neurons of the CeA.
The effect of CRF inhibition was specific to binge-like ethanol consumption. Silencing of CRF-neurons, like CRF-receptor signaling inhibition, had no effect on sucrose consumption. This suggests that the effects of CRF-neuronal inhibition by DREADDs was specific to the pharmacological effects of ethanol and not likely its appetitive traits (Bachmanov, Tordoff, & Beauchamp, 1996). Moreover, the lack of effect on sucrose consumption indicates that CRF-neuronal inhibition in the CeA does not affect natural rewards (Olsen, 2011). No changes in locomotor activity or anxiety-like behavior were observed in the open-field tests (Porsolt, Anton, Blavet, & Jalfre, 1978; Prut & Belzung, 2003). Together, the lack of locomotor and anxiogenic effects reflect a specificity of CRF-neuronal inhibition to alter maladaptations associated with heavy ethanol consumption with little off target effects, a crucial characteristic in therapeutic considerations (Jupp & Lawrence, 2010). Despite the fact that natural rewards were not impacted, it is very likely that Gi/o -DREADD manipulation in the CeA would also alter intake of other drugs of abuse previously shown to be under the maintenance of CRF-signaling including nicotine (Bruijnzeel et al., 2012; Marcinkiewcz et al., 2009), cocaine (Erb, Salmaso, Rodaros, & Stewart, 2001), and other addictive substances (Koob, 2008a, 2008b). It is important to denote that these studies were only done in male mice without considering sex as a biological variable. Recent work suggests that the neuroplastic upregulation in CRF in the CeA may be specific to male mice (Agoglia, Tella, & Herman, 2020; Agoglia et al., 2021). Importantly, the reduction in voluntary consumption after inhibiting CRF1 signaling was seen in both sexes (Agoglia et al., 2021), but the current study and its interpretation only extends to male mice. Additional studies should delineate the sex differences of the impact silencing of CRF neurons on ethanol consumption (Schwandt et al., 2016).
Previous reports show that Gi/o -DREADDs in CRF neurons functionally inhibits activity of CRF+ cells in vitro (Pleil et al., 2015), but herein, the function of Gi/o -DREADDs in CRF neurons was analyzed using c-Fos as previous studies have shown ethanol administration causes upregulation of c-Fos in the CeA (Leriche, Mendez, Zimmer, & Berod, 2008; McBride, 2002; Morales, Criado, Sanna, Henriksen, & Bloom, 1998; Robinson et al., 2020). CNO administration prior to IP ethanol reduced the number of c-Fos+ cells within the CeA suggesting that Gi/o -DREADD activation inhibited CRF-neuronal activity stemming from an ethanol injection. This was expected as electrophysiological evidence indicated that Gi/o -DREADD activation in CRF+ neurons inhibits neuronal activation (Pleil et al., 2015), and that the converse action of activating the Gq-DREADD increased c-Fos expression (Pomrenze et al., 2015). Moreover, this study confirms the fidelity of DREADD expression in CRF neurons in the CeA of CRF-ires-Cre mice as DREADDs were colocalized with an anti-CRF antibody (Andreoli, Marketkar, & Dimitrov, 2017; Walker, Cornish, Lawrence, & Campbell, 2019). However Reduced c-Fos in the CeA induced by Gi/o -DREADD activation coupled with the colocalization of anti-CRF antibody with DREADD confirms that our observation of reduced ethanol consumption, herein, was mediated by selective inhibition of CRF-neurons.
Neuronal-inhibition of CRF-positive cells within the CeA curbed excessive ethanol consumption. Future studies should examine the specific pathways that underlie the effect within the CeA, as it could be mediated by projections to the LHb, BNST or VTA (Companion et al., in press; McBride, 2002; Rinker et al., 2017). By inactivating the CRF-neurons, we may be reducing consumption by preventing ethanol’s disinhibition properties as CeA CRF-neurons co-express GABA (Gilpin et al., 2015; M. Roberto et al., 2010). Regardless of the specific mechanism, these data concur with a growing body of pre-clinical evidence that CRF antagonists are a promising therapeutic avenue for the treatment of AUDs (Higley, Koob, & Mason, 2012; Lowery & Thiele, 2010), especially since polymorphisms in CRH-R1, the human analog of the rodent CRF-R1, has been shown to be involved with excessive ethanol consumption (Blomeyer et al., 2008; Treutlein et al., 2006).
Acknowledgments:
The authors thank Timothy P. Gilliam and Rhiannon D. Thomas for their technical assistance. This research was funded by the following NIH grant: AA022048, AA013573, AA015148, AA021611, AA011605, GM139696, & GM000678.
Footnotes
Conflict of interest statement: The authors declare no competing financial interests. Dr. Thiele owns shares of Glauser Life Sciences, a copy the aims to develop therapeutics for mental health disorders. The work that is presented in this paper is not directly related to the scientific aims of Glauser Life Sciences.
5. References
- Agoglia AE, Tella J, & Herman MA (2020). Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology, 180, 108296. doi: 10.1016/j.neuropharm.2020.108296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Zhu M, Quadir SG, Bluitt MN, Douglass E, Hanback T, . . . Herman MA (2021). Sex-specific plasticity in CRF regulation of inhibitory control in central amygdala CRF1 neurons after chronic voluntary alcohol drinking. Addict Biol, e13067. doi: 10.1111/adb.13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli M, Marketkar T, & Dimitrov E (2017). Contribution of amygdala CRF neurons to chronic pain. Exp Neurol, 298(Pt A), 1–12. doi: 10.1016/j.expneurol.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, & Beauchamp GK (1996). Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res, 20(2), 201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2017). Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology, 122, 115–126. doi: 10.1016/j.neuropharm.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, & Laucht M (2008). Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry, 63(2), 146–151. doi: 10.1016/j.biopsych.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Borrego MB, Grigsby KB, Townsley KG, Chan A, Firsick EJ, Tran A, . . . Ozburn AR (2022). Central nucleus of the amygdala projections onto the nucleus accumbens core regulate binge-like alcohol drinking in a CRF-dependent manner. Neuropharmacology, 203, 108874. doi: 10.1016/j.neuropharm.2021.108874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, & Brewer RD (2011). Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med, 41(5), 516–524. doi: 10.1016/j.amepre.2011.06.045 [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, & Heilig M (2011). Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther, 129(2), 149–171. doi: 10.1016/j.pharmthera.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, & Alexander JC (2012). Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav, 101(1), 62–68. doi: 10.1016/j.pbb.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Companion MA, Gonzalez DA, Robinson SL, Herman MA, & Thiele TE (in press). Lateral habenula-projecting central amygdala circuits expressing GABA and NPY Y1 receptor modualate binge-like ethanol intake in mice. Addiction Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, . . . Thiele TE (2013). Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res, 37(10), 1688–1695. doi: 10.1111/acer.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, & Vetreno RP (2015). Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res, 37(2), 331–341, 344–351. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26695754 [PMC free article] [PubMed] [Google Scholar]
- Curley DE, Webb AE, Sheffler DJ, & Haass-Koffler CL (2021). Corticotropin Releasing Factor Binding Protein as a Novel Target to Restore Brain Homeostasis: Lessons Learned From Alcohol Use Disorder Research. Front Behav Neurosci, 15, 786855. doi: 10.3389/fnbeh.2021.786855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ Jr., & Akil H (1999). Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol, 413(1), 113–128. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10464374 [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, . . . George O (2019). Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun, 10(1), 1238. doi: 10.1038/s41467-019-09183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, & Chen A (2018). The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiological Reviews, 98(4), 2225–2286. doi: 10.1152/physrev.00042.2017 [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, & Stewart J (2001). A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl), 158(4), 360–365. doi: 10.1007/s002130000642 [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, & Thiele TE (2004). Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res, 28(10), 1459–1468. doi: 10.1097/01.alc.0000141809.53115.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Robins MT, Caruso MA, & Ryabinin AE (2021). Social Housing Leads to Increased Ethanol Intake in Male Mice Housed in Environmentally Enriched Cages. Frontiers in Behavioral Neuroscience, 15. Retrieved from https://www.frontiersin.org/article/10.3389/fnbeh.2021.695409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, & Hommer DW (2008). Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci, 28(18), 4583–4591. doi: 10.1523/jneurosci.0086-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, & Roberto M (2015). The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry, 77(10), 859–869. doi: 10.1016/j.biopsych.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, & Sher KJ (2007). Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res, 31(6), 928–938. doi: 10.1111/j.1530-0277.2007.00378.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, & Dautzenberg FM (2006). Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets, 5(4), 453–479. doi: 10.2174/187152706777950684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Koob GF, & Mason BJ (2012). Treatment of alcohol dependence with drug antagonists of the stress response. Alcohol Res, 34(4), 516–521. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23584117 [PMC free article] [PubMed] [Google Scholar]
- Holgate JY, Garcia H, Chatterjee S, & Bartlett SE (2017). Social and environmental enrichment has different effects on ethanol and sucrose consumption in mice. Brain Behav, 7(8), e00767–e00767. doi: 10.1002/brb3.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, & Pickel VM (2009). Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience, 159(2), 526–539. doi: 10.1016/j.neuroscience.2008.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, & Lawrence AJ (2010). New horizons for therapeutics in drug and alcohol abuse. Pharmacol Ther, 125(1), 138–168. doi: 10.1016/j.pharmthera.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Kono J, Konno K, Talukder AH, Fuse T, Abe M, Uchida K, . . . Itoi K (2017). Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Structure and Function, 222(4), 1705–1732. doi: 10.1007/s00429-016-1303-0 [DOI] [PubMed] [Google Scholar]
- Koob GF (2008a). Alcoholism, corticotropin-releasing factor, and molecular genetic allostasis. Biol Psychiatry, 63(2), 137–138. doi: 10.1016/j.biopsych.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2008b). Hedonic Homeostatic Dysregulation as a Driver of Drug-Seeking Behavior. Drug Discov Today Dis Models, 5(4), 207–215. doi: 10.1016/j.ddmod.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifeldt M, Herman MA, Sidhu H, Okhuarobo A, Macedo GC, Shahryari R, . . . Contet C (2022). Central amygdala corticotropin-releasing factor neurons promote hyponeophagia but do not control alcohol drinking in mice. Mol Psychiatry. doi: 10.1038/s41380-022-01496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, & Price JL (1978). Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol, 178(2), 225–254. doi: 10.1002/cne.901780204 [DOI] [PubMed] [Google Scholar]
- Lee HM, Giguere PM, & Roth BL (2014). DREADDs: novel tools for drug discovery and development. Drug Discov Today, 19(4), 469–473. doi: 10.1016/j.drudis.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche M, Mendez M, Zimmer L, & Berod A (2008). Acute ethanol induces Fos in GABAergic and non-GABAergic forebrain neurons: a double-labeling study in the medial prefrontal cortex and extended amygdala. Neuroscience, 153(1), 259–267. doi: 10.1016/j.neuroscience.2008.01.069 [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, . . . Thiele TE (2012). Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci, 32(10), 3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, & Thiele TE (2010). CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology, 35(6), 1241–1252. doi: 10.1038/npp.2009.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, & Thiele TE (2008). The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res, 32(2), 240–248. doi: 10.1111/j.1530-0277.2007.00573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, & Thiele TE (2010). Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets, 9(1), 77–86. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20201818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, & Bruijnzeel AW (2009). Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology, 34(7), 1743–1752. doi: 10.1038/npp.2008.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, & Thiele TE (2015). Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcohol Clin Exp Res, 39(8), 1406–1416. doi: 10.1111/acer.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ (2002). Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav, 71(3), 509–515. doi: 10.1016/s0091-3057(01)00680-3 [DOI] [PubMed] [Google Scholar]
- Morales M, Criado JR, Sanna PP, Henriksen SJ, & Bloom FE (1998). Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res, 798(1–2), 333–336. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9666163 [DOI] [PubMed] [Google Scholar]
- NIAAA. (2004). NIAAA Council Approves Definition of Binge Drinking. National Institute on Alcohol Abuse and Alcoholism Newsletter. [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, & Siggins GR (2009). Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal, 9, 68–85. doi: 10.1100/tsw.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. (2011). Guide for the care and use of laboratory animals (8th ed.). Washington, D.C.: National Academies Press. [PubMed] [Google Scholar]
- Olsen CM (2011). Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology, 61(7), 1109–1122. doi: 10.1016/j.neuropharm.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano M, & Pandey SC (2017). Epigenetic mechanisms of alcoholism and stress-related disorders. Alcohol, 60, 7–18. doi: 10.1016/j.alcohol.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GW, C. (2009). The Rat Brain in Stereotaxic Coordinates (Compact 6th ed.). New York: Academic Press. [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, . . . Kash TL (2015). NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci, 18(4), 545–552. doi: 10.1038/nn.3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, . . . Messing RO (2015). A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front Neurosci, 9, 487. doi: 10.3389/fnins.2015.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, & Jalfre M (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol, 47(4), 379–391. doi: 10.1016/0014-2999(78)90118-8 [DOI] [PubMed] [Google Scholar]
- Prut L, & Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol, 463(1–3), 3–33. doi: 10.1016/s0014-2999(03)01272-x [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav, 84(1), 53–63. doi: 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, . . . Thiele TE (2017). Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry, 81(11), 930–940. doi: 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, . . . Parsons LH (2010). Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry, 67(9), 831–839. doi: 10.1016/j.biopsych.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, & Siggins GR (2012). The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med, 2(12), a012195. doi: 10.1101/cshperspect.a012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Kirson D, & Khom S (2021). The Role of the Central Amygdala in Alcohol Dependence. Cold Spring Harb Perspect Med, 11(2). doi: 10.1101/cshperspect.a039339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, & Varodayan FP (2017). Synaptic targets: Chronic alcohol actions. Neuropharmacology, 122, 85–99. doi: 10.1016/j.neuropharm.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Dornellas APS, Burnham NW, Houck CA, Luhn KL, Bendrath SC, . . . Thiele TE (2020). Distinct and Overlapping Patterns of Acute Ethanol-Induced C-Fos Activation in Two Inbred Replicate Lines of Mice Selected for Drinking to High Blood Ethanol Concentrations. Brain Sci, 10(12). doi: 10.3390/brainsci10120988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, . . . Heilig M (2016). The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology, 41(12), 2818–2829. doi: 10.1038/npp.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan FA, Eldred LM, & Davis DV (2014). Addiction, drinking behavior, and driving under the influence. Subst Use Misuse, 49(6), 661–676. doi: 10.3109/10826084.2013.858167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, & Mahler SV (2016). DREADDS: Use and application in behavioral neuroscience. Behav Neurosci, 130(2), 137–155. doi: 10.1037/bne0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Fee JR, Hayes DM, Knapp DJ, MacNeil DJ, & Thiele TE (2004). Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice. Alcohol Clin Exp Res, 28(9), 1324–1330. doi: 10.1097/01.alc.0000139829.67958.1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, & Thiele TE (2012). The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav, 106(3), 325–331. doi: 10.1016/j.physbeh.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahre MA, Brewer RD, Fonseca VP, & Naimi TS (2009). Binge drinking among U.S. active-duty military personnel. Am J Prev Med, 36(3), 208–217. doi: 10.1016/j.amepre.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48(3), 235–241. doi: 10.1016/j.alcohol.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, . . . Schumann G (2006). Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry, 11(6), 594–602. doi: 10.1038/sj.mp.4001813 [DOI] [PubMed] [Google Scholar]
- Treweek JB, Jaferi A, Colago EE, Zhou P, & Pickel VM (2009). Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. J Comp Neurol, 512(3), 323–335. doi: 10.1002/cne.21884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Okumura T, Shibasaki T, & Sakanaka M (1992). Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res, 577(1), 175–179. doi: 10.1016/0006-8993(92)90554-m [DOI] [PubMed] [Google Scholar]
- Walker LC, Cornish LC, Lawrence AJ, & Campbell EJ (2019). The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: A validation study. Neuropharmacology, 154, 96–106. doi: 10.1016/j.neuropharm.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, & Tache Y (2011). Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res, 1415, 34–46. doi: 10.1016/j.brainres.2011.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersielis KR, Wicks B, Simko H, Cohen SR, Khantsis S, Baksh N, . . . Bangasser DA (2016). Sex differences in corticotropin releasing factor-evoked behavior and activated networks. Psychoneuroendocrinology, 73, 204–216. doi: 10.1016/j.psyneuen.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Gessa GL, & Kreek MJ (2013). Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats. Neurosci Lett, 554, 110–114. doi: 10.1016/j.neulet.2013.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]


