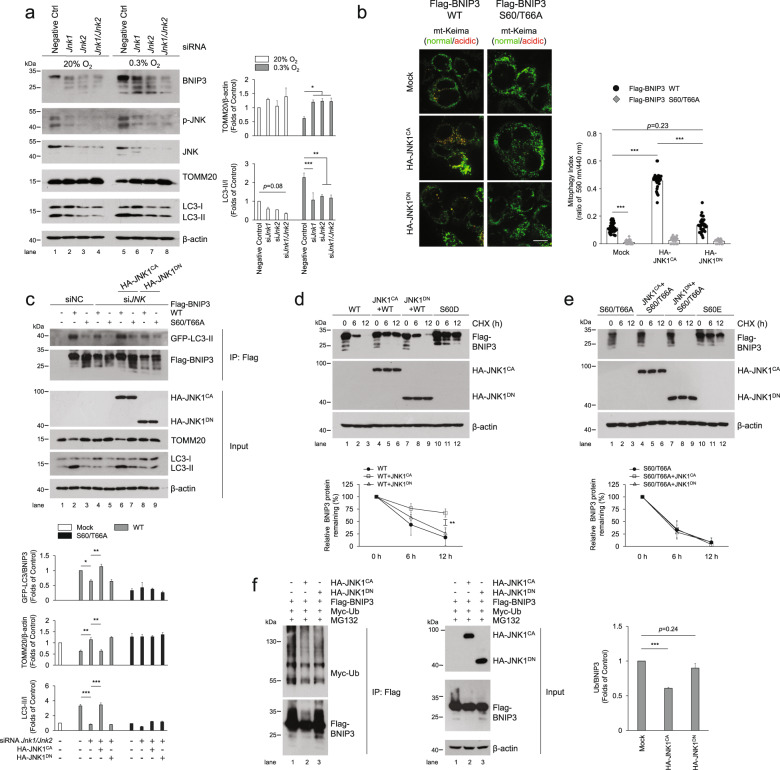
Fig. 6. Phosphorylation of BNIP3 at S60/T66 by JNK enhances mitophagy and impedes BNIP3 proteasomal degradation.
a PC12 cells were transfected with negative control (NC), Jnk1 and Jnk2 siRNA for 48 h and then exposed to 20% O2 or 0.3% O2 for 6 h, and cell lysates were subjected to western blot analysis with the indicated antibodies. n = 3. b HeLa Cells stably expressing mt-Keima were co-transfected with plasmids encoding constitutively active (CA) or dominant negative (DN) HA-JNK1 and Flag-BNIP3 WT or S60/T666A, and mitophagy was identified and quantified by the ratio of acidic (590 nm, red) to normal mitochondria (440 nm, green). Scale bar, 10 μm. n ≧ 30. c HeLa cells were transfected with NC or JNK1 and JNK2 siRNA and plasmids encoding GFP-LC3. After 48 h, the cells were transfected with plasmids encoding wild-type (WT) or S60/66 A Flag-BNIP3 and HA-JNK1CA or HA-JNK1DN for an additional 24 h. Cell lysates were immunoprecipitated with an anti-Flag antibody and examined via western blotting with the indicated antibodies. n = 3. d, e HeLa cells were co-transfected with constitutively active or dominant negative HA-JNK1 and BNIP3 WT (d, left) or the S60/T66A mutants (e, right). After transfection for 48 h, 20 μg ml−1 CHX was added to the cultures for the indicated time, and the degradation of BNIP3 was detected via western blotting with the indicated antibodies and quantified, respectively. f HeLa cells were co-transfected with Flag-BNIP3, Myc-Ub and constitutively active or dominant negative HA-JNK1 for 48 h, and 10 μM MG132 was added 12 h before samples were collected. Cell lysates were boiled and immunoprecipitated with an anti-Flag antibody. The immune complexes were then analyzed via western blotting. n = 3. The data are expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus the indicated group.