Abstract
The global trade in used vehicles and their components generates huge financial benefits but leads to detrimental environmental consequences including groundwater pollution and potential adverse health effects mediated by free-radical processes such as lipid peroxidation. We investigated oxidative stress responses in thirty-six, female mice orally exposed (via drinking) to graded concentrations (0%, 50%, and 100%) of groundwater from a well located within a major automobile junk market in SW-Nigeria containing extremely high levels of arsenic (0.332 ± 0.089 mg/l) and seventeen PAHs, which serves as domestic water supply. Blood samples from the mice were assayed for selected biochemical parameters at intervals of 7, 14, and 28 days. A significant dose- and duration-dependent increase in malondialdehyde (MDA) and Myeloperoxidase (MPO) confirmed oxidative stress onset due to exposure to the polluted well-water, while a significant decline in nitric oxide (NO−) levels may suggest impaired endothelial smooth-muscle relaxation which may lead to the development of metabolic diseases over time. Superoxide dismutase (SOD) and reduced glutathione (GSH) showed a contrasting trend with Glutathione peroxidase (GPx), while Glutathione-S-Transferase (GST) declined significantly by the 28th day. Two clusters were identified by principal component analysis—one involving MDA, SOD, and GSH suggesting that antioxidant responses driven mainly by SOD and GSH proved insufficient in scavenging the free radicals generated by lipid peroxidation. NO− and total protein clustered together possibly due to the significant declines in both over the study period. Histological examination of liver tissue of exposed mice corroborated the above findings and highlights the need for urgent remedial action.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01305-w.
Keywords: Oxidative stress, Anti-oxidants, Groundwater, Automobile junk, Toxicity, Mice
Introduction
Following the wave of global economic recession, automobile wastes, particularly knockdown engines, are continually transferred from industrialized nations to developing countries for recycling and reuse (Nwachukwu et al. 2013). A recent UNEP report put the value of the global trade in used vehicles at USD17.1b in 2014 (UNEP 2020) and Nigerians are reported to have spent an equivalent of USD9.5m on used vehicles in the last 10 years (Oji 2022). As new and/or genuine automobile spare parts become more and more expensive or unavailable, motorists in developing countries have resorted to the use of fairly used parts sold in automobile junk markets. Automobile junk markets generate huge financial benefits, but with adverse health and environmental hazards, due to the improper management of environmental pollution arising from the sale of these automobile parts (Nwachukwu et al. 2013).
Activities associated with the sale of these moderately used automobile parts often involve the disposal of spent oil composed of all fluids including engine oil, brake, transmission, and power steering fluids, used gear lubricants, and hydraulic and transformer oils amongst others. These products are collected during the process of locomotive oil change and from spills during the dismantling of the mechanical components. Typical constituents of engine oil include heavy metals such as lead, cadmium, chromium, arsenic, and zinc (Abdul Zali et al. 2015), as well as paraffinic, naphthenic, and aromatic hydrocarbon fractions (Aljabiri 2018). Spent oil from used vehicles contains even higher concentrations of these pollutants due to increasing wear and tear during the lifecycle of the engines (Pelitli et al. 2017).
In areas where indiscriminate disposal of spent oil occurs, water from precipitation will seep through permeable soil and continue downward, carrying contaminants into the groundwater aquifer; depending on physical and chemical processes such as sorption–desorption, advection, precipitation-dissolution, acid–base, and redox reactions (Postigo et al. 2018). The spilled oil may also be carried along with surface run-off into depressions located close to abstraction wells, thereby resulting in the pollution of these water sources (Nwachukwu et al. 2010). Oral intake of the polluted well-water may elicit adverse health effects in exposed human and animal populations because contamination of water supplies rarely entails acute poisoning but rather a gradual and progressive impairment of health sequel to chronic low-dose exposures (Bondy and Campbell 2018).
Reactive oxygen species (ROS) are produced by the metabolism of normal cells and may be beneficial or harmful depending on the balance between pro-oxidants and antioxidants (Muriel 2009). They are involved in signal transduction; defense against bacteria, parasites, and toxins; and the promotion of environmental resilience through certain genetic mutations that enable adaptation to environmental change. However, the existence of excessive oxygen free radicals also leads to damage to normal cells and tissues (Zhu et al. 2012). Many pollutants are initiators of free radical–mediated processes such as lipid peroxidation, and the oxidative destruction of proteins and DNA. Free radicals, such as superoxide (O2 −), hydroxyl (OH·), and hydroperoxyl (HOO·) radicals are often generated by all initiators (Denisov and Afanas’ev, 2005) and can be used as biomarkers of oxidative stress.
Oxidative stress refers to the imbalance between the production of free radicals and antioxidant defense systems in the body in favor of pro-oxidants (Muriel 2009). It has been linked with several diseases including cardiovascular, neurodegenerative diseases, and diabetes, amongst others (Muriel 2009; Zhu et al. 2012). The metabolism of xenobiotic pollutants may lead to the additional formation of reactive oxygen species (Lackner 1998) increasing pro-oxidant levels in the body. Environmental contaminants, therefore, play an important role in the modulation or alteration of tissue redox homeostasis and as such, highly reactive free radicals generated by toxicants can predispose tissues to oxidative injury (Olayinka and Ore 2015). Enzymatic and non-enzymatic mechanisms are mediated by antioxidants in attenuating the level of pro-oxidants (Zhu et al. 2012). The active oxygen-scavenging enzymatic systems include superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and glutathione S-transferase (GST); while the non-enzymatic antioxidants include glutathione (GSH), vitamins C and E, carotenoids, natural flavonoids, and melatonin, as well as other compounds such as urate and bilirubin (Marí et al. 2010; Zhu et al. 2012).
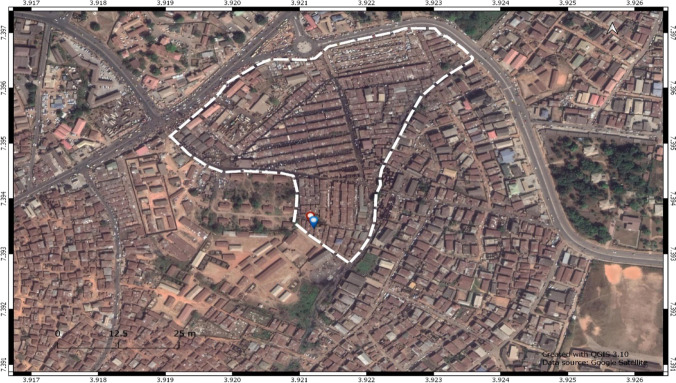
The Araromi automobile junk market serves as a major hub for the sales and repair of automobile replacement parts in Ibadan, South-Western Nigeria. At the market, unserviceable used vehicles are dismantled into parts, which are thereafter sold after any necessary repairs are made. The market has been in operation for over 40 years and generates large volumes of spent oil from automobile waste oil discharge or spillage during the dismantling, servicing, and/or repairs of the vehicular components. The soils are darkened in color due to the indiscriminate discharge of spent oil and other lubricants. In addition, activities such as the riving of engines, welding, iron bending, scraping, and painting take place at the site. This could have implications not only for the groundwater quality of the site but also for human and animal populations in the vicinity of the site who may be exposed particularly via the oral intake of these polluted water sources either through cooking with or drinking contaminated/polluted well-water. The aim of this study was therefore to assess the possible effects polluted groundwater from the site may elicit in orally exposed populations. Due to the biological similarities with humans and the crucial role oxidative stress plays in the onset of several metabolic diseases, we used Swiss albino mice as a mammalian model organism and investigated oxidative stress responses as a possible marker of toxicity in mice exposed to polluted water from the site. The mice were used as models to simulate possible effects in the orally exposed human populations. We focused on groundwater from a shallow well commonly used by residents and traders in food preparation (Fig. 1; Online Resource Plate 1). This well was observed at a later sampling date than seven other wells (at the same site) earlier sampled and analyzed in other studies by our research group. As a result, this well was not included with those sampled in our studies on the alterations in reproductive indices in mice exposed to contaminated urban groundwater from the site (Oni et al. 2019), as well as in our studies on the non-carcinogenic and carcinogenic risks associated with heavy metals and polycyclic aromatic hydrocarbons in well-water samples from the site (Oni et al. 2022).
Fig. 1.
Map of Ibadan metropolis showing the Araromi automobile junk market (inset is the study well—red dot; adjacent to it is the local food outlet—blue dot)
This is important because the pollutant levels observed in the well sampled in this study were between 1 and 260 times higher than the highest levels of the same pollutants observed in the seven other wells previously sampled at the site (Oni et al, 2022). Therefore, the pollutant levels in this well represent a worst-case scenario and it will likely pose much greater risks to the exposed population than was highlighted in our previous studies. Consequently, this well was selected as the focal point of this study. The proximity of this well to a nearby local food outlet and the frequent usage of water from the well in cooking suggests it could be a major ingestion route for contaminated/polluted groundwater in the exposed population. This could in turn predispose exposed individuals to adverse health effects on prolonged usage of these polluted water sources. This, therefore, informed the need for further studies on the possible adverse effects that may be elicited in the exposed population. Consequently, we used a rodent model to investigate oxidative stress responses as a possible marker of toxicity in mice exposed to groundwater from the study well. In selecting a rodent model, we chose mice rather than rats due to their small size and the fact that they cost less to maintain (Bryda 2013). In addition to the biochemical endpoints, we also assessed the histopathology of the liver as an additional marker of toxicity in exposed mice.
Materials and methods
Study area
The Araromi automobile junk market, Ibadan, is located in Ibadan North-East Local Government Area of Oyo State, Nigeria, between latitudes 7° 23′ 35″ N to 7° 23′49″ N and longitudes 3° 55′ 8″ E to 3° 55′23″ E. The well of focus for this study is located on latitudes 7°23′36″7′ N and longitudes 3°55′16″2′ E and at an elevation of 201 m, height above sea level. Nearby is the local food joint on latitudes 7° 23′ 36″4′ N and longitudes 3° 55′16″2′ E (Fig. 1; Online Resource Plate 1).
Water sample collection and analysis
A total quantity of 20L of water (10L each sampling day) was collected from the study well with the aid of a bailer into pre-washed polyethylene containers on the 2nd and 16th of July 2018. In the laboratory, both 10-L samples were mixed to form a composite sample. Heavy metals (lead—Pb, cadmium—Cd, chromium—Cr, mercury—Hg, and arsenic—As) in composite samples were analyzed using Atomic Absorption Spectrometry (AAS), after nitric acid digestion of the samples as recommended by the American Public Health Association APHA (2005). Samples for Polycyclic Aromatic Hydrocarbons (PAHs) analysis were collected into separate 500-ml brown Winchester bottles and analyzed according to the United States Environmental Protection Agency, USEPA (1984) method with slight modification as follows: dichloromethane was used instead of methylene chloride, while hexane was used instead of pentane. Analysis was carried out in triplicate for heavy metals and duplicate for PAHs. Seventeen PAHs were analyzed, which were as follows: 1-methylnaphthalene, 2-methylnaphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benz(a)anthracene, Chrysene, Benzo(b)fluoranthene, Benzo(a)pyrene, Benzo(k)fluoranthene, Indeno(1,2,3-cd)pyrene, Dibenzo(a,h)anthracene, and Benzo(g,h,i)perylene. Composite samples were used in further experiments involving the mice.
Animal selection and care
Thirty-six female mice (Mus musculus) (6–8 weeks old) were purchased from the experimental animal unit of the Department of Zoology, University of Ibadan, Ibadan, and acclimatized to laboratory conditions (room temperature with a 12/12 h light/dark cycle) for 2 weeks before the commencement of the study. Animals were contained in cages made of high-density polyethylene (HDPE) material (dimension: 13.49″L × 10.98″W × 6.58″H) with wire-mesh lids and provided with feed and water ad libitum. The feed provided was a commercial mice diet produced by Ladokun® Feeds, Nigeria Ltd., Ibadan, Nigeria. Cages were cleaned daily and bedded with wood shavings thrice a week. Ethical approval obtained from the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan, Ibadan, under the approval number UI-ACUREC/18/0083 ensured that the research was carried out in line with the guide for the care and use of laboratory animals as stipulated by the National Institute of Health (NIH-85–23 1985).
Experimental design
Three concentrations (0%, 50%, and 100%) of the water treatment were prepared by measuring appropriate volumes of the well-mixed composite well-water sample and distilled water control respectively using a 500-mL measuring cylinder. The 0% concentration was made up of 250 mL of distilled water only, while for the 50% concentration group, equal volumes of the well-mixed well-water and distilled water (125 ml of each) were measured to make a final volume of 250 ml for administration to the test mice. The 50% concentration solution was thereafter homogenized by mixing before administration to the test mice. The 100% concentration was made up of 250 mL of the thoroughly mixed well-water. The control and test samples were administered as drinking water to the mice. Each concentration was renewed daily. The mice were randomly assigned to the experimental groups. Each group was composed of 12 mice. However, the mice in each group were housed in three cages containing four mice each. This was to avoid the stress associated with overcrowding and prevent anti-social behavior. The mice were exposed to the various concentrations for a total of 28 days. Blood samples were collected from the mice at intervals of 7, 14, and 28 days after the commencement of the treatment for the determination of the biochemical parameters. Four mice were sacrificed from each of the experimental groups at each time interval. The values presented represent Means ± SD.
Blood sampling and serum extraction
Before sacrifice, about 2 ml of blood was obtained from the retro-orbital plexus of each mouse using non-heparinized capillary tubes on days 7, 14, and 28 after the commencement of the test water administration. Blood samples were allowed to coagulate for 30 min, after which they were centrifuged at 3000 rpm for 10 min as described by Onyema et al. (2006) using a UNICO PowerSpin™ LX C858E centrifuge. The serum obtained (the supernatant) was separated from the coagulated blood using a micropipette and stored in a freezer at − 4 °C before the commencement of the biochemical assays.
Biochemical analyses
Lipid peroxidation (determination of malondialdehyde concentration): Serum MDA concentration was determined according to the method described by Yoshioka et al. (1979). The absorbance of the clear supernatant was measured against n-butanol at 535 nm using a spectrophotometer (model SM23A). MDA concentration of serum sample was evaluated from the standard curve of 1,1,3,3 tetra ethoxy propane and results were presented as μmol/l. The serum myeloperoxidase (MPO) activity was determined according to the method of Xia and Zweier (1997). The change in absorbance at 460 nm was measured using a spectrophotometer (Perkin-Elmer MmPF44B). One unit (U) of MPO activity was defined as that degrading 1 μmol of H2O2 per minute at 25 °C. The nitric oxide (NO) was measured as described by Olaleye et al. (2007) using the Griess reaction. The absorbance at 550 nm was measured using a spectrophotometer (Perkin-Elmer MmPF44B). The concentration of nitrite in the sample was determined from a sodium nitrite (NaNO2) standard curve and was expressed as micromole nitrite per milliliter.
Superoxide dismutase (SOD) activity was determined according to the method described by Sun et al. (1988). The SOD activity was monitored at 560 nm by detecting the inhibition of the nitroblue tetrazolium reduction rate using a spectrophotometer (model SM23A). SOD activity was expressed as U/l. Serum Glutathione peroxidase (GPx) activity was determined according to the procedure proposed by Paglia and Valentine (1967). The absorbance change was monitored at 340 nm for 5 min at 37 °C using a spectrophotometer and GPx activity was expressed as nmol GSH oxidized per min per ml. The antioxidant capacity of reduced glutathione (GSH) was quantified following the procedure given by Hu (1994). The emission intensity at 420 nm was measured at an excitation of 350 nm using a spectrophotometer (Perkin-Elmer MmPF44B). The Glutathione-S-transferase (GST) activity was estimated according to the method of Habig et al. (1974). This involved the production of a complex formed from the enzymatic conjugation of reduced glutathione with the aromatic substrate, 1-chloro-2, 4 nitrobenzene (CDNB), which was measured using a spectrophotometer (Perkin-Elmer MmPF44B) at an absorbance of 280 nm. Enzyme activity was measured as the nmole of CDNB-GSH complex formed per min per ml. The serum protein concentration was determined by the method of Gornal et al. (1949). The reaction mixture was thereafter read with a spectrophotometer (Perkin-Elmer MmPF44B) at 540 nm using distilled water as blank.
Histopathology
After blood sample collection, mice were sacrificed by cervical dislocation and dissected. Thereafter, the liver was excised, sectioned, and fixed in universal bottles containing 10% formalin solution. The tissue samples were pre-embedded to allow the infiltration and replacement of the water content of the tissues with paraffin wax. Thereafter, the tissue sections were dehydrated in graded solutions of ethanol and then embedded in paraffin and sectioned to 5 µm using a microtome. Tissue sections were stained with hematoxylin and eosin (H & E) (Junquiera and Carneiro 2011) cleared in xylene, mounted on glass slides, and viewed under a microscope. Histopathological procedures were in line with the protocols described by Morton et al. (1997) and Slaoui and Fiette (2011).
Data presentation and statistical analysis
Data were presented as mean ± standard deviation (SD) of four mice for each of the three concentration groups and exposure periods. Statistical significance was determined by a two-way analysis of variance (ANOVA). Means of data collected were compared using Duncan’s multiple range test on IBM SPSS statistical package 20.0. Means with similar letters as indicated in the results are not significant at the 5% level. The oxidative stress responses (the dependent variables) in mice in the three different experimental groups (the independent variables) were subjected to principal component analysis (PCA). Principal component analysis (PCA) is a commonly used multivariate technique carried out on the response of the dependent variables in a multivariate dataset (Syms, 2008). In essence, the PCA is used to reduce the dimensionality of the data to summarize the most important or defining parts, while simultaneously filtering out the noise (Moore et al., 2006). Patterns in PCA are solely a function of relationships between the dependent variables (Syms, 2008).
First, the entire dataset on the oxidative stress responses in the mice in the three experimental groups on days 7, 14, and 28 (i.e., days 7, 14, and 28 data combined) were subjected to the PCA. In addition, the dataset on all stress response parameters for mice in the three groups for each day was also considered and analyzed separately using the PCA. The acceptable criteria for PCA, i.e., Overall Kaiser–Meyer–Olkin measure of sampling adequacy > 0.7, Bartlett’s test of Sphericity with p-value < 0.001, and the correlation matrix determinant value > 0.00001 in the four scenarios: (the entire dataset for days 7, 14 and 28 combined; and day 7, 14 and 28 data separately) was then compared. Kaiser–Meyer–Olkin refers to the calculated measures of the entire correlation matrix and each individual variable which evaluates the appropriateness of applying PCA (Jolliffe 2002). Variables with individual measures of sampling adequacy of above 0.5 which are considered acceptable (Wuensch, 2012) were used in the PCA. Based on these criteria, MPO was excluded due to its low measure of sampling adequacy. Therefore, only seven of the eight oxidative stress responses (variables) were used for the PCA. We focused on the scenario that gave the best fit for the PCA based on all the above criteria, the patterns of which are explained in the discussion. The statistical package IBM SPSS version 20.0 was used to perform the PCA.
Results
Water analysis
Heavy metals
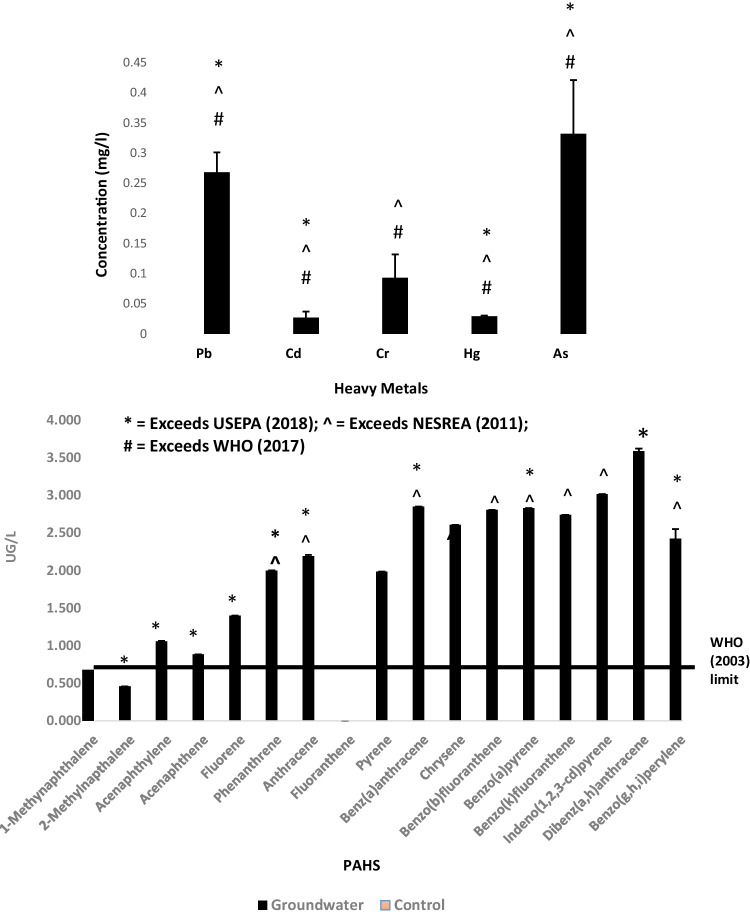
Lead, cadmium, chromium, mercury, and arsenic were identified in groundwater from the study well with values ranging from 0.03 ± 0.01 mg/L for cadmium to 0.33 ± 0.09 mg/L for arsenic. Metals in the groundwater followed the order: As > Pb > Cr > Cd = Hg. Metal levels in the distilled water control were however below detection limits (Fig. 2).
Fig. 2.
Heavy metal and PAH concentrations in the groundwater and distilled water control sample. Error bars indicate SD; n = 3 for heavy metals; n = 2 for PAHs. Values for the distilled water control were below detection limits
Polycyclic aromatic hydrocarbons (PAHs)
Sixteen PAHs were identified in groundwater from the study well. These were as follows: 1-Methylnaphthalene, 2-Methyl-naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Pyrene, Benzo(a)Anthracene, Chrysene, Benzo(b)Fluoranthene, Benzo (a) Pyrene, Benzo (k) Fluoranthene, Indeno(1, 2, 3-cd)pyrene, Dibenzo(a, h)anthracene, and Benzo(g,h,i)perylene. Fluoranthene was not detected in the groundwater. The values obtained ranged from 0.46 ± 0.00 µg/l (2-Methylnaphthalene) to 3.59 ± 0.00 µg/l (Dibenzo(a,h)anthracene) (Fig. 2).
Oxidative stress biomarkers
Lipid peroxidation
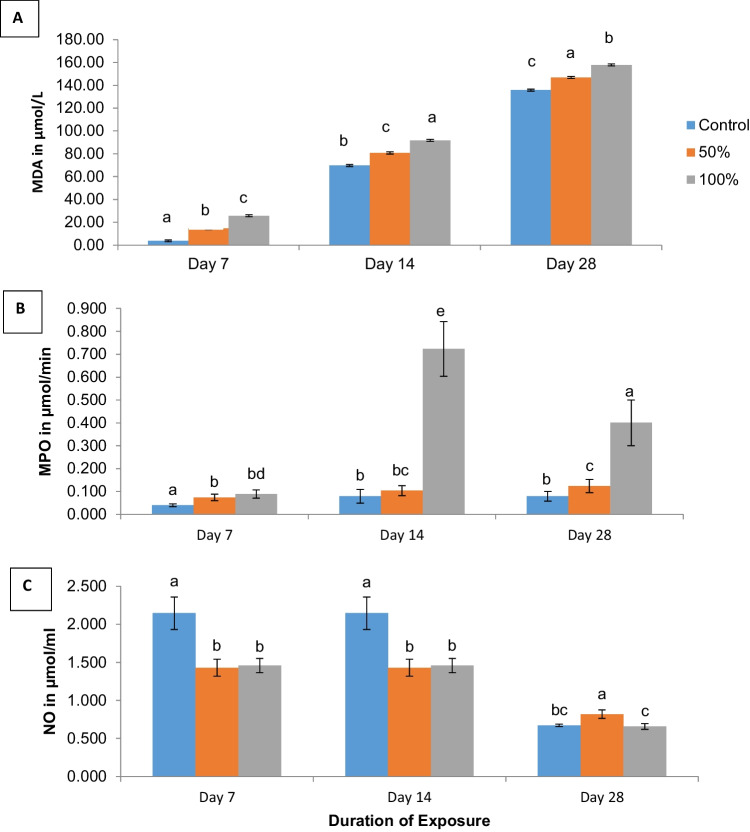
The effect of pro-oxidants as shown by malondialdehyde (MDA) levels indicated a dose-dependent increase in lipid peroxidation between mice exposed to the control, 50% and 100% of the test water on days 7, 14, and 28 respectively. Furthermore, the differences observed between the test concentrations and exposure duration were statistically significant at p < 0.05 (Fig. 3A).
Fig. 3.
A–C Mean MDA, MPO, and NO levels in serum of mice exposed to well-water from Araromi Automobile junk market, Ibadan. Means with the same letters are not significantly different at p < 0.05. Error bars indicate SD; n = 4
Myeloperoxidase (MPO) activity
At 7 days, myeloperoxidase (MPO) activity increased in a concentration-dependent pattern. (Control: 0.039 ± 0.01, 50%: 0.074 ± 0.01 and 100%: 0.088 ± 0.02 µmol / min). A similar trend was also observed in MPO activity at 14 days exposure period with values of 0.079 ± 0.03, 0.103 ± 0.02, and 0.723 ± 0.12 µmol/min respectively. The same pattern was observed at 28 days. In all instances, mean MPO activity in the mice increased significantly (p < 0.05) across the various concentration groups, as well as the exposure periods (Fig. 3B).
Nitric oxide (NO)
Mean nitric oxide (NO) levels in mice exposed to 50% and 100% of the test water were significantly lower at 1.43 ± 0.11 and 1.46 ± 0.09 µmol/ml respectively when compared with nitric oxide levels in the control (2.15 ± 0.21 µmol/ml) at the 7th and 14th day exposure period (Fig. 3C). In contrast, by the 28th day, mean nitric oxide levels in mice exposed to 50% of the test water were significantly elevated (p < 0.05) at 0.82 ± 0.06 µmol/ml, when compared to levels in control mice (0.67 ± 0.02) µmol/min and the 100% concentration group (0.66 ± 0.04) µmol/ml). There was however no significant difference (p < 0.05) in mean NO levels of mice exposed to 100% of the test water when compared to control mice at 28 days of exposure. The overall values for NO in all test groups on day 28 were nonetheless significantly lower (p < 0.05) compared to levels obtained on days 7 and 14 respectively (Fig. 3C).
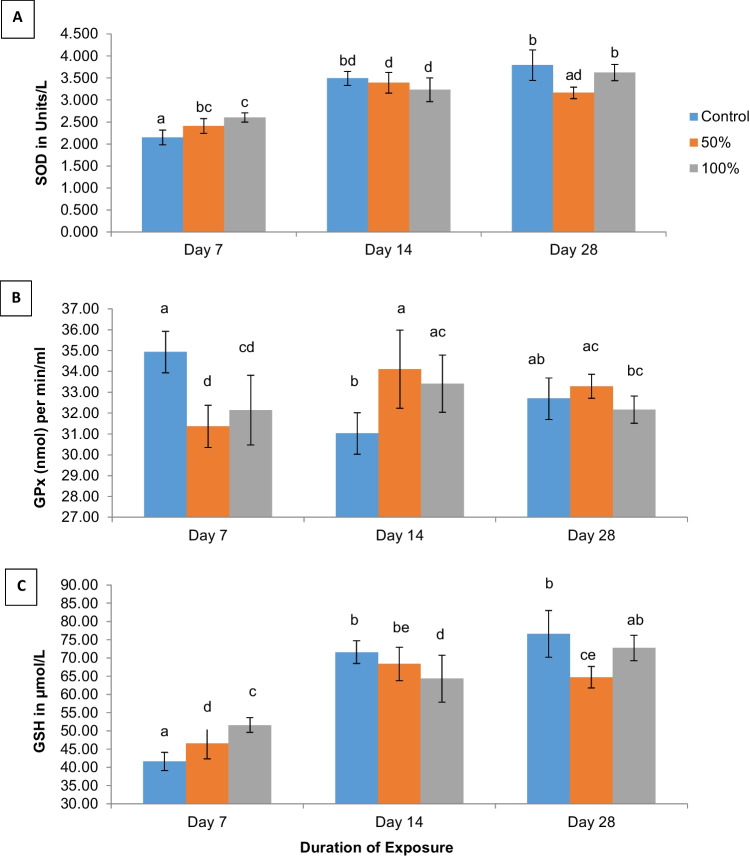
SOD activity
SOD activity showed a significant (p < 0.05) concentration-dependent increase in treatment groups compared to the control at 7 days of exposure, and a concentration-dependent but non-significant (p < 0.05) decrease at 14 days of exposure (Fig. 4A). At 28 days of exposure, SOD activity in mice exposed to 50% of the test water was significantly lower (p < 0.05) at 3.16 ± 0.13 units/L compared to activity in control mice (3.79 ± 0.35 units/L) and the 100% concentration group (3.62 ± 0.18 units/L) which did not differ significantly from each other at p < 0.05 (Fig. 4A).
Fig. 4.
A–C Comparison of mean SOD, GPx, and GSH levels in serum of mice exposed to well-water from Araromi Automobile junk parts market, Ibadan. Means with the same letters are not significantly different at p < 0.05. Error bars indicate SD; n = 4
GPx activity
By the 7th day, mean GPx activity in mice exposed to 50% (31.37 ± 1.00 nmol) and 100% (32.14 ± 1.70 nmol) of the test water was significantly lower (p < 0.05) compared to mean levels in control mice (34.93 ± 1.90 nmol) (Fig. 4B). In contrast, at 14 days, serum GPx activity in mice exposed to both concentration levels increased significantly (p < 0.05) to 34.11 ± 1.90 and 33.42 ± 1.40 nmol respectively, when compared to the control (31.02 ± 1.00 nmol). Similarly, by the 28th day, mean GPx activity in mice exposed to 50% of the test water reduced to 33.28 ± 0.60 nmol, although levels were still elevated when compared to the control mice (32.70 nmol) and the 100% concentration group (32.17 ± 0.70 nmol). However, mean GPx levels in mice in all three concentrations on day 28 were not statistically different at p < 0.05 (Fig. 4B).
GSH activity
The reduced glutathione tripeptide (GSH) activity increased significantly (p < 0.05) in a concentration-dependent pattern at 7 days of exposure. However, a concentration-dependent decrease in serum GSH activity was observed at 14 days of exposure, with the decrease being significant at p < 0.05 in the 100% concentration group (Fig. 4C). Similarly on day 14, there was no significant difference (p < 0.05) in mean GSH activity between mice exposed to the control (71.57 ± 3.10) and the 50% concentration group (68.37 ± 4.59) µmol/L respectively. On day 28, mean GSH activity was significantly reduced in mice exposed to the 50% test concentration (64.71 ± 2.99 µmol/L), while GSH activity in mice exposed to 100% of the test water (72.77 ± 3.49 µmol/L) did not differ significantly (p < 0.05) from mean GSH levels in the control (76.59 ± 6.42 µmol/L). There was a statistically significant difference in mean GSH activity between the exposure durations at p < 0.05 (Fig. 4C).
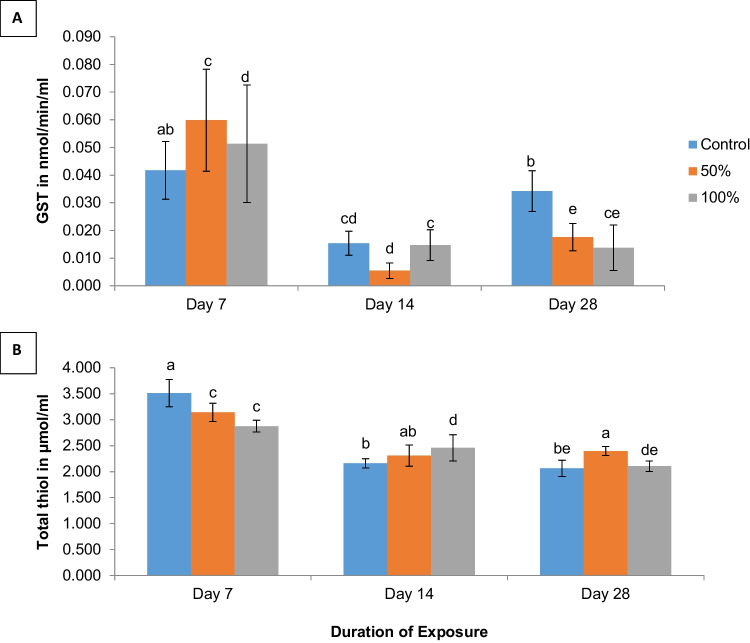
GST activity
There was a significant increase (p < 0.05) in serum GST activity in the treatment groups (50%: 0.06 ± 0.02 and 100%: 0.05 ± 0.02 nmol/min/mL) at 7 days, compared to mice in the control group (0.04 ± 0.01 nmol/min/mL) (Fig. 5A). On the 14th day, GST activity in mice exposed to 50% of the test water decreased compared to the control, although this was not significant at p < 0.05. However, it differed significantly from serum GST activity in the 100% concentration group, which in turn was statistically similar to that of the control (Fig. 5A). Serum GST activity decreased significantly (p < 0.05) in the treatment groups (50%: 0.023 ± 0.005 and 100%: 0.028 ± 0.008 nmol/min/mL) compared to the control (0.034 ± 0.007 nmol/min/mL) by the 28th day. There was a statistically significant difference between the exposure durations at p < 0.05 (Fig. 5A).
Fig. 5.
A–B Comparison of mean GST and Total thiol levels in serum of mice exposed to well-water and distilled water control (0%) from Araromi Automobile junk market, Ibadan. Means with the same letters are not significantly different at p < 0.05. Error bars indicate SD; n = 4
Total thiol
Total thiol levels followed a dose-dependent pattern, decreasing significantly with increased concentration of the polluted water at 7 days of exposure. On the other hand, total thiol levels increased by the 14th day in mice in the control and treated groups at 2.16 ± 0.09, 2.31 ± 0.20, and 2.46 ± 0.25 µmol/mL respectively, with the increase significant at the p < 0.05 level in the 100% concentration group. By the 28th day of exposure, total thiol levels in mice exposed to 50% and 100% (2.40 ± 0.09 and 2.11 ± 0.10 µmol/mL respectively) of the test water were higher compared to levels in control mice (2.06 ± 0.16 µmol/mL), although this difference was only significant at p < 0.05 for the 50% concentration group (Fig. 5B). There was a statistically significant difference between the exposure durations at p < 0.05.
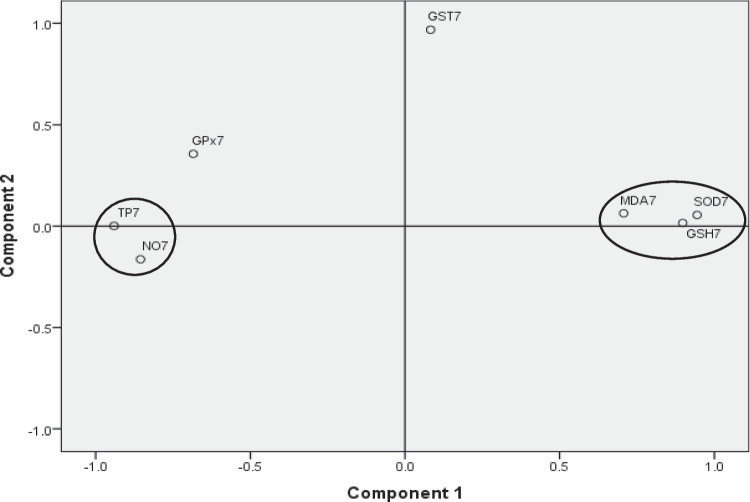
Principal component analysis (PCA)
Results of the PCA showed that only day 7 data gave the best results based on the specified criteria: Kaiser–Meyer–Olkin measure of sampling adequacy value above 0.7 (0.713), Bartlett’s test of Sphericity with a p-value of 0.000 which is less than the benchmark of 0.05 and therefore significant. All other scenarios gave a KMO below 0.7. Therefore, we focused on the patterns highlighted by PCA analysis of day 7 data which best explained the relationship between the dependent variables, i.e., the oxidative responses observed in the mice in the various groups. Two factors were extracted which explained 77% of the variance with the first factor explaining 61% of the variance, while the second factor explained an additional 16% of the variance. The first factor had strong positive loadings for SOD (0.944), GSH (0.898), and MDA (0.707), while total protein (− 0.941), nitric oxide (− 0.855), and GPx (− 0.685) loaded negatively on the same principal component. The second principal component had only one variable, GST (0.968) loading strongly and positively on it (Table 1). Two clusters were identified in the component plot. The first cluster involved MDA, SOD, and GSH, while the second cluster involved total protein and nitric oxide (Fig. 6).
Table 1.
Rotated component matrix for the PCA
| Component | ||
|---|---|---|
| 1 | 2 | |
| gpx7 | − .685 | .357 |
| gsh7 | .898 | |
| gst7 | .968 | |
| mda7 | .707 | |
| no7 | − .855 | |
| sod7 | .944 | |
| tp7 | − .941 | |
Fig. 6.
Component plot for the PCA for the oxidative stress markers
Histopathology
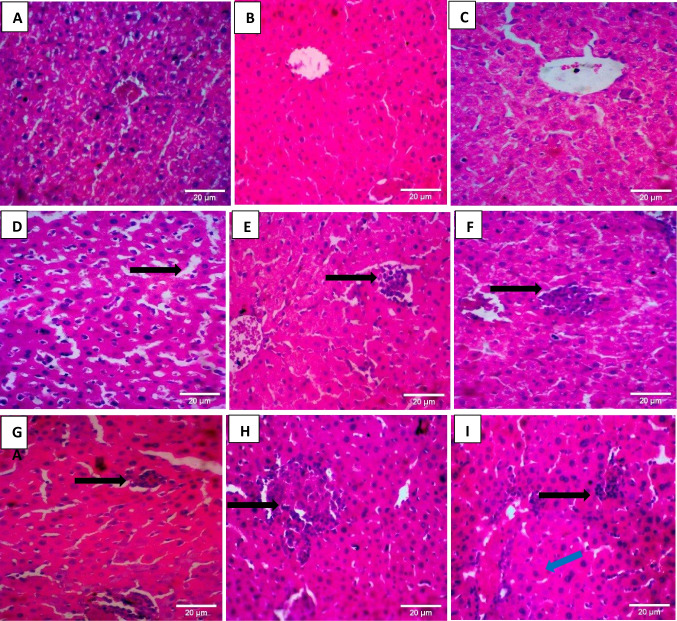
No visible lesions were observed in the liver of control mice on days 7, 14, and 28 of exposure to the contaminated water. However, lesions such as dilation of sinusoids, mononuclear cellular infiltration, hepatocellular necrosis, and Kupffer cell hyperplasia were observed in mice exposed to 50% and 100% of the contaminated water, and this followed a dose-dependent pattern, with mice at the highest concentration showing the greatest severity in the lesions observed (Fig. 7A–I).
Fig. 7.
Photomicrographs of liver tissues of control and exposed mice on days 7, 14, and 28 respectively. A–C (Control—NVL = no visible lesion). D–F (50% well-water; D mild dilation of sinusoids; E mononuclear cellular infiltration (multiple foci); F megalocyte and hepatocellular necrosis—few foci). G–I (100% well-water; mononuclear cellular infiltration—black arrows; diffuse Kupffer cell hyperplasia—blue arrow)
Discussion
Water analysis
The well-water used in this study has been established to be polluted with heavy metals and polycyclic aromatic hydrocarbons (PAHs), attributed to the indiscriminate disposal of spent oils from automobile scrap sale and repair activities in the study location. Mean levels of mercury (0.029 ± 0.0006 mg/L), arsenic (0.332 ± 0.089 mg/L), cadmium (0.027 ± 0.010 mg/L), and lead (0.268 ± 0.033 mg/L) in the well-water exceeded the local regulatory limits of Nigeria’s National Environmental Standards and Regulations Enforcement Agency—NESREA (i.e., Hg: 0.00005, As: 0.010, Cd: 0.0004, and Pb: 0.015 mg/L—NESREA 2011). Similarly, all the analyzed metals exceeded the regulatory limits of the World Health Organization (Hg: 0.006, As: 0.010, Cr: 0.050, Cd: 0.0004, and Pb: 0.015 mg/L—WHO, 2017) and the United States Environmental Protection Agency (Hg: 0.002, As: 0.010, Cd: 0.005, Pb: 0.000 mg/L) except for chromium (0.093 ± 0.039 mg/L) which was within the USEPA limit of 0.100 mg/L (USEPA, 2018). In addition, all the sixteen PAHs identified in the well-water exceeded the WHO (2003) limit of 0.7 µg/L, while ten and twelve of them exceeded the various limits given by NESREA (2011) and USEPA (2018) respectively. Three of these: [benzo (a) pyrene, benzo (a) anthracene, and chrysene] are listed as carcinogens according to Abdel-Shafy and Mansour (2016).
It is important to note that while lead (0.268 ± 0.033 mg/L) and cadmium (0.027 ± 0.010 mg/L) levels in the well-water in this study were only marginally higher than the highest levels of similar metals observed in water from seven other wells from the same site (Pb: 0.20 ± 0.17 mg/L; Cd: 0.02 ± 0.03 mg/L), arsenic and PAHs levels in well-water in this study ranged between 17 and 261 times higher than in the previously sampled wells and indicates much higher health risks than were suggested in the previous study on the carcinogenic and non-carcinogenic risks associated with heavy metals and PAHs in well-water of the site (Oni et al. 2022). This is indicative of severe degradation of the groundwater quality due to activities associated with automobile spare-parts sale and repair activities in the study area, with grave health implications for the exposed population who utilize water from this well for cooking, drinking, and other domestic purposes.
Oxidative stress biomarkers
Malondialdehyde formation is a by-product of lipid hydro-peroxide decomposition. The significant increase in MDA observed in the serum of the mice with increased concentration of polluted water and duration of exposure is suggestive of the increased production of free radicals and lipid peroxidation. Lipid peroxide is formed by oxidation; thus, the higher the serum lipid peroxide levels, the stronger the oxidative stress (Naito et al. 2010). The rise in MDA levels in the control over the study period may be explained by the fact that ROS are by-products of normal cell activity, produced in many cellular compartments, and play a major role in many signaling pathways (Snezhkina et al. 2019). However, priority pollutants such as polycyclic aromatic hydrocarbons and metals like cadmium, mercury, and lead when present at levels above regulatory limits may lead to additional free radical generation resulting in lipid peroxidation, DNA damage, and depletion of protein sulfhydryl amongst other effects (Lackner 1998; Tunegová et al. 2016; Briffa et al. 2020). This may ultimately result in metabolic disorders over time, as evidence has shown that lipid peroxidation of unsaturated fatty acids is involved in the onset and progression of many pathologies such as cardiovascular and neurodegenerative diseases (Sultana et al. 2013).
Myeloperoxidase, an enzyme linked to both inflammation and oxidative stress (Trilianty et al. 2016), showed a concentration-dependent increase with increasing concentration of the polluted water and corroborates the elevated MDA levels observed in the serum of exposed mice. It is secreted at inflammatory sites where it catalyzes the reaction between hydrogen peroxide and halide anions such as chloride to produce a very reactive compound, hypochlorous acid (HOCl), which is a primary oxidant (Sugiyama et al. 2001) that may also be a major contributor to the oxidative stress responses observed. The increased MPO activity, therefore, suggests an increased production of pro-oxidants which may impart a state of oxidative stress. MPO-mediated oxidative damage may also result in an increased likelihood of diseases such as atherosclerosis and related vascular diseases, as well as Parkinson’s and Alzheimer’s disease as observed in experimental animals (Frijhoff et al. 2015).
Nitric oxide levels were highest in mice exposed to the distilled water control and significantly reduced in mice exposed to the 50% and 100% test concentrations of the polluted water after 7 and 14 days, with much lower concentrations by day 28. This implies that increasing concentration of polluted water was associated with increased levels of biomarkers of lipid peroxidation and MPO indicative of oxidative stress, and a corresponding reduction in NO. Nitric oxide has been found to play an important role in smooth muscle relaxation. It is essential in the maintenance of normal endothelial function and under conditions promoting the formation of ROS, NO bioavailability is decreased. Thus, increased intake of the polluted water produced a downregulation of NO available for normal vasodilation. The reduction in nitric oxide (NO) with increasing concentration of the polluted water may not be unconnected with its reaction with a free radical, the superoxide radical (O2−) to form a very potent oxidant and reactive nitrogen species, peroxynitrite (ONOO−); the anionic form of which may, in turn, react with carbon dioxide present in cells at millimolar concentrations to form another very reactive adduct, nitrosoperoxycarbonate (ONOOCO2−). These reactive adducts may further decay to form other more potent and oxidizing radicals such as OH and CO3−, thus contributing to the oxidative stress responses observed in exposed mice. Peroxynitrite can also react with DNA to form 8-hydroxy-2-deoxyguanosine (8-OHdG) and 8-nitroguanine in cells which can lead to cytotoxicity (Kalyanaraman 2013; Sultana et al. 2013).
The decreasing levels of nitric oxide with increased concentration of polluted water may also be attributed to the very high levels of arsenic and to a lesser extent, lead in the water. Arsenic has been shown to induce alterations in the metabolism of nitric oxide and endothelial function in people who ingest high doses of arsenic in drinking water. Lead may also result in the depletion of nitric oxide (Briffa et al. 2020). The mean value for arsenic in the groundwater in this study (0.332 ± 0.089 mg/l) was 33 times greater than Nigeria’s NESREA, WHO, and USEPA’s regulatory limit of 0.01, while lead levels were about 18 to 27 times higher than local and international regulatory limits suggesting high doses of arsenic (and to a lesser extent lead) in the well-water on ingestion by the mice may have induced alterations in nitric oxide metabolism. The downregulation of NO leading to declining levels in exposed mice may ultimately result in compromised endothelial smooth muscle relaxation suggesting that similar effects may occur in orally exposed individuals thereby predisposing them to a greater risk of developing cardiovascular diseases such as hypertension on prolonged exposure to the polluted water. Hypertension caused by lead has been observed in rats accompanying depleted nitric oxide levels (Briffa et al, 2020). Decreased NO levels are therefore associated with cardiovascular, neurodegenerative, and other chronic inflammatory diseases (Murad 2006; Kalyanaraman 2013) and may also contribute to the underlying cause of impotence and erectile dysfunction. Rajfer et al. (1992) and Kalyanaraman (2013) reported impaired relaxation of the smooth muscle of the corpus cavernosum due to a reduction in NO levels. In a previous study (Oni et al., 2019), mice exposed to increasing concentrations of polluted water from the site (not inclusive of the well sampled in this study) showed significantly reduced sperm count and motility, as well as significantly elevated levels of luteinizing and follicle-stimulating hormone in the 100% concentration after 84-day exposure period, suggesting that pollutants in the water also induce reproductive toxicity in exposed mice, perhaps through similar mechanisms involving a reduction in NO.
Antioxidant defense mechanisms, including SOD, GSH, and GPx, amongst others, are also recognized molecular biomarkers for environmental toxicology, important because of their involvement in the protection of cells against free radicals produced during oxidative stress (Mañas et al. 2013). The rise in SOD and the corresponding decline in GPx levels in the control and exposed mice by day 7 may be due to the overexpression of SOD activity relative to that of GPx, which may lead to a net increase in the generation of hydrogen peroxide. SOD is one of the enzymes classified as the first line of antioxidant defenses due to its rapid pro-oxidant neutralizing potential (Ighodaro and Akinloye 2017) and it catalyzes the dismutation of the superoxide radical (O2−) to produce oxygen and H2O2. Increased production of SOD thus reflects a high production of superoxide radicals (O2−), which in turn react rapidly with molecules of each other (self-dismutation reaction) to produce H2O2. The decline in the levels of GPx in exposed mice by day 7 may not be unconnected with its role in the possible detoxification of the H2O2 produced by the catalytic dismutation of the superoxide radical (O2−). Glutathione peroxidases (GPxs) are a family of selenium-dependent isozymes that catalyze the reduction of H2O2 or organic hydroperoxides to water and alcohols through the oxidation of GSH to GSSG, in this way detoxifying H2O2 and other lipid peroxides to the corresponding alcohol (Kalyanaraman 2013). GSH, a tripeptide and the most abundant non-protein thiol present in cells (Mañas et al. 2013), is, therefore, a determining substrate of the anti-oxidative activity of GPx in mice (Marí et al. 2010).
GSH showed a dose-dependent increase in both control and test animals on day 7. The predominantly higher levels of GSH would suggest its decreased oxidation to GSSG as is expected during detoxification reactions of GPx. This may be suggestive of an inability of GPx to adequately detoxify H2O2, formed from the dismutation reactions of the superoxide radicals catalyzed by SOD and hence clear up the free radicals generated during oxidative stress. The steady and sustained rise in lipid peroxides during this period may suggest that the antioxidant response was insufficient, probably due to redox reactions between heavy metals present in the groundwater with the hydrogen peroxide to generate the toxic and highly reactive free hydroxyl radicals through the Haber–Weiss/Fenton reaction, thus contributing to oxidative stress and possibly to oxidative damage to biological molecules including nucleic acids (Sultana et al., 2013). Therefore, it is possible that the SOD-catalyzed dismutation reaction may have resulted in a net increase in hydrogen peroxide which could have led to the harmful generation of the hydroxyl radical through the Haber–Weiss and Fenton reactions (Kalyanaraman 2013; García-Ruiz and Fernández-Checa 2018), thus contributing to the oxidative stress response.
In contrast, the marginal but non-significant decline in SOD levels in exposed mice on days 14 and 28 implies less SOD is available to catalyze the dismutation reaction of the superoxide radical to form hydrogen peroxide. This may make the superoxide radical free to react with nitric oxide leading to the formation of the highly reactive nitrogen species peroxynitrite (ONOO−) which may in turn ultimately lead to the formation of more potent free radicals such as the OH radical thus contributing to oxidative stress and lipid peroxidation. The significant rise in GPx levels and the corresponding decline in GSH in exposed mice by day 14 are probably illustrative of the anti-oxidative activity of GPx in detoxifying the organic hydroperoxides. This assertion is also supported by the steady decline in GSH levels observed on day 14 implying its possible oxidation to GSSG and confirming the anti-oxidative activity of GPx. However, the fact that the mice had been exposed to increasing concentration of the polluted water for a longer period by days 14 and 28 may also suggest increased free radical generation arising from pollutants in the well-water. Despite the attempts of the antioxidant enzymes such as GPx at clearing these free radicals, the dose-dependent increase in MDA and MPO levels indicates that these enzymes were unable to effectively clear up the free radicals leading to excess oxidant activity and consequently oxidative stress. It is particularly noteworthy the pattern of a rise in the levels of antioxidants SOD, GSH, and GST in exposed mice relative to control mice by day 7, followed by a decline in the exposed groups compared to the control by days 14 and 28. This pattern may be attributed to an initial rise in the antioxidant response to clear up the free radicals generated owing to oxidative stress, followed by inhibition in the activity of these antioxidant enzymes.
The significant reduction in GSH in exposed mice by days 14 and 28 may also be due to the formation of complexes with glutathione by pollutants in the water. Alissa and Ferns (2011) and Trilianty et al. (2016) showed that certain contaminants in the groundwater such as mercury may form complexes with glutathione, thus depleting the availability of the GSH cellular defenses against oxidation. The direct conjugation of lead with glutathione may also lead to the depletion of GSH. Glutathione is also a necessary component for arsenic metabolism (Alissa and Ferns 2011) and considering the high levels of arsenic present in the water, it is not unsurprising that glutathione cellular defenses may be depleted. GST levels have also been widely used as a biomarker of pollution response. Current literature indicates that chronic exposure to environmental pollutants may increase or decrease the activity of GST (Bocedi et al. 2019). The initial increase in GST levels observed in exposed mice by day 7 could be due to an overexpression of GST in a bid to protect against ROS. However, the subsequent decline in GST levels in exposed mice thus suggests that GST is indeed a good biomarker of groundwater contaminants that inhibit GST’s ability to detoxify xenobiotics (Bocedi et al. 2019). The initial rise in GST in exposed mice may also be explained by the role of GST as an important phase II conjugating enzyme which assists phase I conjugating enzymes such as the cytochrome P450 mixed-function oxidases (MFOs) to bio-transform many relatively insoluble organic compounds such as PAHs in the water into more water-soluble forms so they can be excreted in bile or urine. Considering the high concentration of PAHs well above regulatory limits, PAHs such as Benzo(a)pyrene may be bio-transformed into chemically reactive forms more toxic than the parent compound which are capable of causing mutations or cancer (Ryan and Hightower 1996). The induction of MFOs by PAHs in the polluted well-water may lead to significant increases in both protein levels and enzymatic activity of the cytochrome P450 MFOs (Ryan and Hightower, 1996), although further studies would be necessary to confirm this.
Low levels of thiols have been observed in various disorders associated with increased generation of free radicals (Muttigi et al. 2009). The observed concentration-dependent decrease in total protein thiols after 7 days could be attributed to the complementary role of protein thiols as essential molecules in the maintenance of redox status. After 14 days, the protein thiols followed an inverse pattern to GSH; thus, as GSH activity increased, the activity of protein thiols was decreased indicating that the protein thiol levels are involved in redox homeostasis relative to the GSH activity in the serum of the mice. In addition, reactive nitrogen species such as peroxynitrite can oxidize protein thiols forming disulfide, and nitrate tyrosyl groups in proteins forming nitrated proteins, ultimately resulting in a loss or gain of function in the activity of the targeted protein (Kalyanaraman 2013; Sultana et al. 2013).
Principal component analysis of the oxidative stress biomarkers
A KMO value indicates how well a dataset is suited to factor analysis with values above 0.7 as acceptable. The results of the PCA may be suggestive of the role of SOD and GSH in the antioxidant response within the first 7 days. The high positive loading of SOD (0.944) and moderate negative loading of GPx (− 0.685) on the first PC probably suggests an overexpression of SOD relative to GPx, which may result in a net increase of H2O2 as explained earlier. The strong positive loading of GSH (0.898) implies its decreased oxidation to GSSG, and attests to the fact that GPx was not effective at detoxifying hydrogen peroxide, thus making it available to react with trace metal contaminants in the water, resulting in the generation of the hydroxyl radical, and thus contributing to increasing lipid peroxidation (as shown by the high positive loading of MDA: 0.707) with increasing concentration and duration of exposure (Kalyanaraman 2013; García-Ruiz and Fernández-Checa 2018). Alternatively, SOD may not effectively catalyze the dismutation reaction of the superoxide (O2−) radical leaving it free to react with nitric oxide (NO) leading to the generation of peroxynitrite (ONOO−), another potent oxidant that may in turn decay to form other potent oxidants such as the hydroxyl and carbonate radicals which may, in turn, contribute to lipid peroxidation. The negative loading of nitric oxide on PC 1 (− 0.855) confirms the declining levels of nitric oxide with increasing free radical generation, and implies the possible reaction of NO with the superoxide radical (O2−) to form peroxynitrite, thus contributing to increased oxidative stress (Kalyanaraman 2013; Sultana et al.2013). The decline in levels of NO may also imply possible impairment of endothelium-dependent vasodilation and smooth muscles of the corpus cavernosum (Rajfer et al. 1992), predisposing exposed individuals to cardiovascular diseases and erectile dysfunction on long-term exposure to polluted water from the study site. The generation of hydrogen peroxide by SOD, which becomes available to facilitate the reaction between MPO and halide anions to form the potent oxidant hypohalous acid, may also contribute to increased oxidative stress (Sugiyama et al. 2001). The negative loading of total protein on PC 1 (− 0.941) confirms that low levels of protein thiols are associated with the increased generation of free radicals and can result in compromised protein function (Kalyanaraman 2013; Sultana et al. 2013). The strong positive loading of GST on PC 2 (0.968) probably suggests additional antioxidant action in an attempt to clear up free radicals generated from oxidative stress. It could also be related to its detoxification role as a phase II enzyme assisting phase I enzymes such as cytochrome P450 MFOs to break down organic compounds such as PAHs in the well-water (Ryan and Hightower, 1996).
Liver histopathology
Due to its unique metabolic functions and its relation to the gastrointestinal tract, the liver is an important target of xenobiotics. Lesions such as sinusoidal dilation were observed in the liver of mice exposed to 50% of the well-water and corroborate the findings on oxidative stress induction by pollutants in the well-water. Hepatic sinusoidal dilatation refers to the enlargement of the hepatic capillaries (Brancatelli et al. 2018). It is a circulatory disturbance alteration that has also been observed in the livers of fish from sites contaminated with crude and dispersed oil and is considered indicative of the impact of the oil on the health of these fish (Myers et al. 1998; Marty et al. 2003; Agamy 2012). In addition, abnormally large cells or megalocytes and necrotic changes were also observed in the liver of exposed mice. Megalocytosis and necrosis have also been reported in the livers of fish treated with the water-soluble fraction of crude and dispersed oil, as well as in fish from crude oil–contaminated sites. Megalocytosis has also been induced in laboratory experiments as a result of exposure to chemicals (Agamy 2012). Sinusoidal dilation, megalocytosis, and the necrotic changes observed in the exposed mice may therefore be due to derivatives of crude oil such as spent engine oil, a major contaminant at the site of this study and suggests that crude oil and its derivatives may induce similar lesions in exposed organisms. The observed lesions may therefore not be unconnected to the presence of heavy metals and PAHs in the spent oil-contaminated well-water.
For instance, the liver has long been identified as a target organ for arsenic exposure (Noman et al. 2015). Methylation of arsenic occurs mostly in the liver (Briffa et al, 2020). Studies by Noman et al. (2015) on arsenic-induced histological alterations in the liver of mice exposed to arsenic through contaminated groundwater showed moderate degeneration and necrosis in the hepatic parenchyma with mild to moderate fatty change, whereas the control did not reveal any lesions of pathological significance. The authors identified increased oxidative stress in tissue due to arsenic exposure as the major cause of arsenic-induced toxicity in the mice (Noman et al. 2015). The high levels of arsenic in the study well, the dose, and duration-dependent increase in MDA, as well as the dose-dependent increase in MPO (Fig. 3), both markers of oxidative stress and the necrosis observed in the exposed mice (Fig. 7F) all corroborate the conclusions of Noman et al. (2015) on oxidative stress being a major mechanism in arsenic-induced toxicity and suggests that the high levels of arsenic in the well-water may likely be a major contributor to the oxidative stress responses and necrosis observed in the exposed mice. However, the effect of other metals such as lead and the PAHs cannot be ruled out.
The toxicity of lead on liver cells is also well established and exposure to it increases oxidative stress that results in liver injury. The combination of organic solvents with lead may also cause injury to the liver due to the similarities in characteristics with lead (Farmand et al. 2005; Malaguarnera et al. 2012; Mitra et al. 2022). Chronic lead exposure is potentially toxic to liver cells, resulting in glycogen depletion and cellular infiltration, which can result in chronic liver injury and cirrhosis (Hegazy and Fouad 2014; Mitra et al. 2022). Although glycogen depletion was not observed in the liver of exposed mice in this study, the increased severity of mononuclear cellular infiltration with increasing concentration of the polluted water (Fig. 7) may be suggestive of the contribution of lead and/or other organic compounds in the polluted water to the observed hepatotoxicity. Unlike circulatory system disturbances such as sinusoid dilation which is a reversible degenerative change that does not alter the normal function of the tissue, degenerative alterations such as hepatocellular necrosis is a more severe cytomorphological abnormality that negatively imparts tissue function and is considered a direct effect of toxicants that are generally irreversible. Their persistence or progression may thus lead to a partial or total loss of organ function (Agamy, 2012). Necrotic changes are categorized as responses to lethal injury from which the organism or cell cannot recover, and they often result when all protective and corrective measures including tier 1 and 2 biomarkers have failed (Ryan and Hightower, 1996).
Suitability of the biomarkers and suggestions for further study
Measurements at any level of biological organization in either wild populations from contaminated habitats or organisms (and cell cultures) experimentally exposed to physical or chemical stressors, which indicate sub-lethal exposures to, or effects of toxic chemicals are known as biomarkers (Ryan and Hightower, 1994). The dose-dependent correlation of the pro-oxidant and antioxidant enzymes and the histological lesions observed in the liver with increasing concentrations of the polluted well-water suggests that they are suitable biomarkers for use in environmental monitoring, both to estimate environmental exposures to toxic chemicals and to detect damage resulting from such exposures. However, for biomarkers to be effectively and reliably used, their requirements and limitations must be understood. Biomarkers must have biological significance and should be more sensitive than conventional endpoints such as growth, survival, or reproductivity. In addition, the molecular level changes should be able to be linked to both cellular and higher level effects (Ryan and Hightower, 1996).
The changes in the pro-oxidant and antioxidant proteins in this study are representative of biomarkers at the lower molecular and cellular levels of organization, while the lesions observed in the histology of the liver are representative of biomarkers at higher (tissue, organ, and whole organism) levels of biological organization. The dose-dependent responses in these biomarkers with stress-induced damage owing to the presence of heavy metal and polycyclic aromatic hydrocarbons in the well-water suggest that these biomarkers have biological significance. The absence of mortality in experimental animals throughout the experiment further indicates that these biomarkers are early indicators and more sensitive than conventional endpoints such as survival, and most likely growth and reproductivity. Since all environmental pollutants begin their harmful or pathological processes at the molecular or subcellular levels, the observed changes in these biomarkers at these levels may thus provide earlier signals that exposure or injury has occurred (Moore et al. 1994; Ryan and Hightower 1996).
Besides the pro-oxidant and antioxidant enzymes, many other stress proteins are used in environmental monitoring to provide evidence that organisms have been exposed to, or affected by xenobiotic chemicals. These include the heat shock proteins, a group of proteins referred to as “molecular chaperones” which have been found to respond to a broad range of chemical, physical, and biological stressors. They assist the processes related to the correct folding of proteins, essentially assisting newly formed proteins to assume the proper configuration to perform their tasks in the cell cycle (Moreira-de-Souza et al. 2018). Reactive oxygen species can cause extensive cysteine modification of proteins, disturbing proteostasis thus activating the heat shock response (HSR) and Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor (Nrf2) pathways via the heat shock transcription factor 1 (Hsf1) and the redox-sensitive transcription factor Nrf2 leading to elevated expression of the heat shock proteins, particularly hsp70s. The hsp70s work as central hubs in the protein quality network to maintain proteostasis and regulate the activity, expression, and degradation of the antioxidant enzymes to contribute to redox homeostasis (Zhang et al. 2022). Hsp70s essentially work to prevent stress-induced damage but if overwhelmed by the stress, they repair it or limit further damage (Ryan and Hightower 1996). Free radical generation during oxidative stress may also have activated the heat shock response proteins which in turn may have played their regulatory activity in controlling the expression of the antioxidant response. However, the fact that the antioxidant response was insufficient to effectively detoxify or clear up the free radicals may suggest that the hsp70 stress proteins which regulate the expression of the antioxidant enzymes may also have been overwhelmed by the pollution stress. Further studies are however necessary to confirm this and it is therefore recommended for future study.
Other biomarkers such as metallothioneins which play a major role in the chelation of heavy metal ions and protection of cells from heavy metal ion-induced damage, as well as the cytochrome P450 MFOs involved in the detoxification of organic contaminants, may also be investigated. In addition, the use of in vitro systems to evaluate potential biomarkers and elucidate the underlying mechanisms that control their responses at the cellular level may also be investigated in further studies. This will complement the in vivo studies and may play an important role in evaluating progress in remediating the polluted area, answering the question, when is it clean enough (Ryan and Hightower 1996).
Conclusion
This study established oxidative stress in mice exposed to polluted water from the Araromi automobile junk market, Ibadan, Nigeria. Lipid peroxidation increased in exposed mice compared to controls over the study period. Although initial elevation of antioxidants occurred in exposed mice, antioxidant defenses proved insufficient to suppress the levels of the pro-oxidants thus predisposing exposed mice to oxidative stress. Histopathological changes observed in the liver of exposed mice compared to normal controls corroborate the observed effects. This report suggests that exposed human and animal populations may be at increased risk of adverse health effects on prolonged usage of the water, especially because pollutant levels in the studied well were much higher than national and international regulatory limits, and were 1 to 260 times higher than values obtained in our earlier studies (Oni et al. 2022). Thus, the toxic effects induced by pollutants in the sample well in this study may be greater. Considering that our earlier studies also indicated that some traders and/or residents had worked or lived at the site for up to 30 years, further studies on the health status of the exposed human population at the site are also recommended. While the influence of other confounding factors such as diet and exercise on toxicity may not be ruled out, such studies may highlight some interesting facts which may corroborate the above findings. Enlightenment campaigns for residents and traders, as well as remediation of groundwater in the study area, are therefore urgently required, while clean potable water should be provided to the community pending cleanup and restoration of the site. Overall, these results highlight the critical role oxidative stress responses play in toxicity following pollutant exposure for sustainable environmental impact.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conceptualization, original draft preparation, formal analysis, writing—review and editing, supervision: Adeola Anike Oni. Methodology, formal analysis, and investigation: Miracle Ogagaoghene Osoh. Methodology, formal analysis, and investigation: Adedayo Opeyemi Obikoya. Methodology, review and editing, supervision: Obokparo Godspower Ohore. All authors read and approved the final article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- [APHA] American Public Health Association (2005) Standard methods for the examination of water and wastewater. 21st Edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
- Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25:107–123. doi: 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- Abdul Zali M, Wan Ahmad WK, Retnam A, Catrina NG. Concentration of heavy metals in virgin, used, recovered and waste oil: a spectroscopic study. Procedia Environ Sci. 2015;30:201–204. doi: 10.1016/j.proenv.2015.10.036. [DOI] [Google Scholar]
- Agamy E. Histopathological changes in the livers of Rabbitfish (Siganus canaliculatus) following exposure to crude oil and dispersed oil. Toxicol Pathol. 2012;40:1128–1140. doi: 10.1177/0192623312448936. [DOI] [PubMed] [Google Scholar]
- Alissa EM, Ferns GA (2011) Heavy metal poisoning and cardiovascular disease. J Toxicol Volume 2011, Article ID 870125, 21 pages. 10.1155/2011/870125 [DOI] [PMC free article] [PubMed]
- Aljabiri NA. A comparative study of recycling used lubricating oils using various methods. J Sci Eng Res. 2018;5(9):168–177. [Google Scholar]
- Bocedi A, Noce A, Marrone G, Noce G, Cattani G, Gambardella G, Lauro MD, Daniele ND, Ricci G. Glutathione transferase P1–1 an enzyme useful in biomedicine and as biomarker in clinical practice and environmental pollution. Nutrients. 2019;11(1741):1–34. doi: 10.3390/nu11081741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SC, Campbell A. Water quality and brain function. Int J Environ Res Public Health. 2018;15(2):1–15. doi: 10.3390/ijerph15010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancatelli G, Furlan A, Calandra A, Burgio MD. Hepatic Sinusoidal Dilatation Abdom Radiol. 2018;43:2011–2022. doi: 10.1007/s00261-018-1465-8. [DOI] [PubMed] [Google Scholar]
- Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects. Heliyon. 2020;6(e04691):1–26. doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryda EC. The mighty mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207–211. [PMC free article] [PubMed] [Google Scholar]
- Denisov ET, Afanas’ev IB (2005) Oxidation and antioxidants in organic chemistry and biology. CRC Press, Taylor and Francis Group, 6000 Broken Sound Parkway NW Boca Raton, FL 33487–2742. 10.1021/ja059854f
- Farmand F, Ehdaje A, Roberts CK, Sindhu RK. Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ Res. 2005;98(1):33–39. doi: 10.1016/j.envres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23(14):1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz C, Fernández-Checa JC. Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol Commun. 2018;2:1425–1439. doi: 10.1002/hep4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornal AG, Bardawill JC, David MM. Determination of serum proteins by means of biuret reaction. J Biol Chem. 1949;177:751–766. doi: 10.1016/S0021-9258(18)57021-6. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jacoby WB. Glutathione-S-transferase activity: the first enzyme step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- Hegazy AMS, Fouad UA. Evaluation of lead hepatotoxicity: histological, histochemical, and ultrastructural study. Forensic Med Anat Res. 2014;2(3):70–79. doi: 10.4236/fmar.2014.23013. [DOI] [Google Scholar]
- Hu ML. Protein thiol groups and glutathione in plasma. Meth Enzymol. 1994;41(233):380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- Ighodaro OM, Akinloye OA (2017) First-line defense antioxidants-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alexandria J Med 1-710.1016/j.ajme.2017.09.001
- Jolliffe IT (2002) Principal component analysis. 2nd Ed. (Springer Series in Statistics). Springer-Verlag New York, Inc.
- Junquiera L, Carneiro J (2011) Basic histology text and atlas. 11th Edition. McGraw Hill Medical. 544
- Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants, and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner R. “Oxidative stress” in fish by environmental pollutants. In: Hinton DE, Streit B, editors. Braunbeck T. Birkhauser Verlag Basel/Switzerland: Fish Ecotoxicology; 1998. pp. 203–224. [Google Scholar]
- Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. 2012;18:2756–2766. doi: 10.3748/wjgv18i22.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañas F, Peralta L, Ugnia L, Weyers A, Garcia Ovando H, Gorla N. Oxidative stress and comet assay in tissues of mice administered glyphosate and AMPA in drinking water for 14 days. J Basic Appl Genet, Volume 24, Issue 2. Article. 2013;7:67–75. [Google Scholar]
- Marí M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernandez-Checa JC. Redox Control of Liver function in health and disease. Antioxid Redox Signal. 2010;12(11):1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev. 2017;6501046:32. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty GD, Hoffman A, Okihiro KH, Hanes D. Retrospective analysis: bile hydrocarbons and histopathology of demersal rockfish in Prince William, Saint Alaska, after the Exxon Valdez oil spill. Mar Environ Res. 2003;56:569–584. doi: 10.1016/S0141-1136(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Mitra S, Chakraboty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, Idris AM, Khandaker MU, Osman H, Alhumaydhi FA. Simal-Gandara J (2022) Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. 2022;34(101865):1–21. doi: 10.1016/j.jksus.2022.101865. [DOI] [Google Scholar]
- Moore MN, Kohler A, Lowe DM, Simpson MG. An integrated approach to cellular biomarkers in fish. In: Fossi MC, Leonzio C, editors. Nondestructive biomarkers in vertebrates. Boca Raton, FL: Lewis Publishers; 1994. pp. 171–197. [Google Scholar]
- Moore MN, Allen JI, McVeigh A. Environmental prognostics: an integrated model supporting lysosomal stress responses as predictive biomarkers of animal health status. Mar Environ Res. 2006;61:278–304. doi: 10.1016/j.marenvres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Moreira-de-Souza C, Bastao de Souza R, Fontanetti CS. HSP70 as a biomarker: an excellent tool in environmental contamination analysis – a review. Water Air Soil Pollut. 2018;229(264):1–12. doi: 10.1007/s11270-018-3920-0. [DOI] [Google Scholar]
- Morton D, Safron JA, Glosson J, Rice DW, Wilson DM, White RD. Histological lesions associated with intravenous saline solution in large volumes of isotonic saline solution in large volumes of isotonic saline solution in rats for 30 days. Toxicol Pathol. 1997;25:390–394. doi: 10.1177/019262339702500407. [DOI] [PubMed] [Google Scholar]
- Murad F. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/nejmsa063904. [DOI] [PubMed] [Google Scholar]
- Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttigi MS, Kedage V, Suvarna R, Rao SS, Joshi C, et al. Serum GST activity and total thiols status in patients with liver disease secondary to various disorders. Open Hepatol J. 2009;1:5–8. [Google Scholar]
- Myers MS, Johnson LL, Olson OP, Stehr CM, Horness BH, Collier TK, McCain BB. Toxicopathic hepatic lesions as biomarkers of chemical contaminant exposure and effects in marine bottom-fish species from the North East and Pacific Coast. Mar Pollut Bull. 1998;37:92–113. doi: 10.1016/S0025-326X(98)00135-0. [DOI] [Google Scholar]
- Naito Y, Lee MC, Kato Y, Nagai R, Yonei Y. Oxidative Stress Markers. Anti-Aging Med. 2010;7(5):36–44. doi: 10.3793/jaam.7.36. [DOI] [Google Scholar]
- [NESREA] National Environmental Standards and Regulations Enforcement Agency. April 28, 2011. National environmental (surface and groundwater quality control) regulations. http://extwprlegs1.fao.org/docs/pdf/nig145947.pdf. Accessed 11 January 2019
- Noman ASM, Dilruba S, Mohanto NC, Rahman L, Khatun Z, Riad W, AlMamun A, Alam S, Aktar S, Chowdhury S, Saud ZA, Rahman Z, Hossain K, Haque A. Arsenic-induced histological alterations in various organs of mice. J Cytol Histol. 2015;6(3):1–13. doi: 10.4172/2157-7099.1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachukwu MA, Feng H, Achilike K. Integrated study for automobile wastes management and environmentally friendly mechanic villages in the Imo River basin. Nigeria Afr J Environ Sci Technol. 2010;4(4):234–249. [Google Scholar]
- Nwachukwu MA, Ntesat B, Mbaneme FC. Assessment of direct soil pollution in automobile junk market. J Environ Chem Ecotox. 2013;5(5):136–146. doi: 10.5897/jece2013.0280. [DOI] [Google Scholar]
- Oji, H (2022) Auto firm’s stocks crash as Nigerians spend N4tr on “Tokunbo” in 10 years. The Guardian, 20Th June 2022.
- Olaleye SB, Adaramoye OA, Erigbali PP, Adeniyi OS. Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol exposed rats. World J Gastroenterol. 2007;13(38):5121–5126. doi: 10.3748/wjg.v13.i38.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayinka ET, Ore A. Hepatotoxicity, nephrotoxicity, and oxidative stress in rat testis following exposure to haloxyfop-p-methyl ester, an aryloxyphenoxypropionate herbicide. Toxics. 2015;3:373–389. doi: 10.3390/toxics3040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni AA, Amama IA, Omole EO, Ohore OG. Alterations in reproductive indices in mice exposed to contaminated urban groundwater from Araromi automobile spare parts market, Ibadan, South-Western Nigeria. Arch Basic Appl Med. 2019;7:67–78. [Google Scholar]
- Oni AA, Babalola SO, Adeleye AD, Olagunju TE, Amama IA, Omole EO. Ohore OG (2022) Non-carcinogenic and carcinogenic risks associated with heavy metals and polycyclic aromatic hydrocarbons in well-water samples from an automobile junk market in Ibadan SW-Nigeria. Heliyon. 2022;8:e10688. doi: 10.1016/j.heliyon.2022.e10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate-induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–24. [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Pelitli V, Doğan Ö, Köroğlum HJ. Waste oil management analyses of waste oils from vehicle crankcases and gearboxes. Global J Environ Sci Manage. 2017;3(1):11–20. doi: 10.22034/gjesm.2017.03.01.002. [DOI] [Google Scholar]
- Postigo C, Martinez DE, Grondona S, Miglioranza KSB (2018) Groundwater pollution: sources, mechanisms, and prevention. The Encyclopedia of the Anthropocene. Eds. D. A., Della Sala and M. I. Goldstein. 5: 87–96. 10.1016/B978-0-12-409548-9.09880-8
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ. Ignarro LJ (1992) Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to non-adrenogenic, non-cholinergic neurotransmission. N Engl J Med. 1992;326:90–94. doi: 10.1056/nejm199201093260203. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Hightower LE. Evaluation of heavy-metal ion toxicity in fish cells using a combined stress protein and cytotoxicity assay. Environ Toxicol Chem. 1994;13(8):1231–1240. doi: 10.1002/etc.5620130804. [DOI] [Google Scholar]
- Ryan JA, Hightower LE. Stress proteins as molecular biomarkers for environmental toxicology. In: Morimoto RI, Yahara I, Polla B, editors. Feige U. Birkhauser Verlag Basel/Switzerland: Stress-inducible Cellular Responses; 1996. pp. 411–424. [DOI] [PubMed] [Google Scholar]
- Slaoui M, Fiette L (2011) Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol Biol 691:69–82. 10.1007/978-1-60761-849-2_4 [DOI] [PubMed]
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS. Dmitriev AA (2019) ROS generation and antioxidant defense system in normal and malignant cells. Oxid Med Cell Longev. 2019;6175804:1–17. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Cenini G, Butterfield DA. Biomarkers of oxidative stress in neurodegenerative diseases. In: Villamena FA, editor. Molecular Basis of Oxidative Stress. John Wiley & Sons Inc; 2013. pp. 359–376. [Google Scholar]
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte-macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–891. doi: 10.1016/s0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. doi: 10.1093/clinchem/34.3.497. [DOI] [PubMed] [Google Scholar]
- Syms C. Principal Component Analysis. In: Jorgensen SE, Fath BD, editors. Encyclopedia of ecology. Oxford: Elsevier; 2008. pp. 2940–2949. [Google Scholar]
- Trilianty L, Alexandra FD, Jelita H, Suhartono E. Myeloperoxidase as an indicator of liver cells inflammation induced by mercury. Int J Pharm Clin Res. 2016;8(11):1516–1521. [Google Scholar]
- Tunegová M, Toman R, Tančin V. Heavy metals – environmental contaminants and their occurrence in different types of milk. Slovak J Anim Sci. 2016;49(3):122–131. [Google Scholar]
- [UNEP] United Nations Environmental Program (2020) Used vehicles and the environment. A global overview of used light-duty vehicles: flow, scale, and regulation. Economy Division. UNEP, Nairobi, Kenya. 108pp. June 2020 https://wedocs.unep.org/20.500.11822/34175
- [USEPA] United States Environmental Protection Agency. March 22, 2018. National primary drinking water regulation. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations Accessed 10 January 2019
- [USEPA] United States Environmental Protection Agency (1984) Method 610: polynuclear aromatic hydrocarbons https://www.epa.gov/sites/default/files/2015-10/documents/method_610_1984.pdf Accessed 25 June 2018
- [WHO] World Health Organization (2003) Polynuclear aromatic hydrocarbons in drinking water. https://www.who.int/water_sanitation_health/publications/water-quality/guidelines/chemicals/polynuclear-aromatic-hydrocarbons-fs-new.pdf Accessed 11 January 2017
- [WHO] World Health Organization (2017) Guidelines for drinking water quality; first addendum to the third edition. https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ Accessed 11 January 2019 [PubMed]
- Wuensch KL (2012) Multivariate analysis with SPSS. core.ecu.edu:core.ecu.edu/psyc/wuenschk/SPSS/SPSS-MV.htm Accessed September 2016
- Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245:93e6. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygens toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gong W, Wu S, Perreti S. Hsp70 in redox homeostasis. Cells. 2022;11(829):1–18. doi: 10.3390/cells11050829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012 doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.