Abstract
The main danger of cold stress to animals in cold regions is systemic metabolic changes and protein synthesis inhibition. RBM3, an exceptional cold shock protein, is rapidly upregulated in response to hypothermia to resist the adverse effects of cold stress. However, the mechanism of the protective effect and the rapid upregulation of RBM3 remains unclear. O-GlcNAcylation, an atypical O-glycosylation, is precisely regulated only by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) and participates in the signal transduction of multiple cellular stress responses as a “stress and nutrition receptor.” Therefore, our study aimed to explore the mechanism of RBM3 regulating glucose metabolism and promoting survival in skeletal muscle under acute cold exposure. Meanwhile, our study verifies whether O-GlcNAcylation mediated by OGT rapidly upregulates RBM3. The blood and skeletal muscle of mice were collected at the end of cold exposure treatment for 0, 2, and 4 h. Changes in levels of RBM3, AKT, glycolysis apoptosis, and OGT were measured. The results show that acute cold exposure upregulated RBM3, OGT, and AKT phosphorylation and increased energy consumption, which enhanced glycolysis and prevent apoptosis. In the 32 °C mild hypothermia model in vitro, overexpression of RBM3 enhanced AKT phosphorylation. Meanwhile, inactivation of AKT by wortmannin resulted in increased apoptosis and decreased glucose metabolism in skeletal muscle under acute cold exposure. In addition, OGT-mediated O-GlcNAcylation of p65 was confirmed in mouse myoblast cell line (C2C12) cells at mild hypothermia. O-GlcNAcylation level affected p65 activity and nuclear translocation. Compared with wild type (WT) mice, RBM3 and p65 phosphorylation were decreased in specific skeletal muscle Ogt (KO) mice, whereas AKT phosphorylation, glycolysis, and apoptosis were increased. Taken together, O-GlcNAcylation of p65 upregulates RBM3 to promote AKT phosphorylation, enhance glucose metabolism, and reduce apoptosis in skeletal muscle of mice under acute cold exposure.
Keywords: RBM3, AKT, Glucose metabolism, O-GlcNAc, p65
Introduction
A cold environment is an inevitable and common stressor in humans and livestock living in cold areas. It destroys the homeostasis of thermostatic animals and leads to specific and non-specific reactions to cold adaptation, such as affecting neuroendocrine, reproduction, and immunity, causing oxidative stress, autophagy, and apoptosis (Messmer et al. 2014; Zhao et al. 2014; Lian et al. 2018). Exposure to low temperatures significantly alters metabolic levels in mammals, increasing food intake, activation of lipid mobilization in brown adipose tissue glucose mobilization, and systemic energy consumption (Hao et al. 2015; Morton et al. 2017; Wu et al. 2020). Among these cold-induced adaptive changes in glucose and fat metabolism, glucose uptake, glycolysis, gluconeogenesis, and glycogen synthesis play key roles in resistance to cold (Sepa-Kishi et al. 2018). Skeletal muscle and brown adipose tissue, two major thermal tissues in mammals, cooperate in response to cold and maintain core body temperature (Blondin et al. 2015). The shivering and (Wellmann et al. 2010; Venugopal et al. 2016; Wong et al. 2016) non-shivering thermogenesis of skeletal muscle induced by cold and its activation of glucose conversion to maintain glucose levels during cold exposure are beneficial to mammalian survival (Pant et al. 2016). In addition, skeletal muscle can also be used as a secretory organ to secrete a variety of polypeptides in response to environmental and metabolic changes, and then crosstalk with fat, liver, and other organs to regulate energy homeostasis (Pedersen and Febbraio 2012; Giudice and Taylor 2017). Cold exposure has an adverse effect on the properties and quality of skeletal muscle (Short 2019).
While the body regulates energy metabolism to make positive changes in response to cold exposure, it also regulates the synthesis of some proteins to correspond with biological processes in homeostasis reconstruction (Kong et al. 2020). Protein synthesis is generally inhibited when mammals develop hypothermia due to cold exposure (Tong et al. 2013). In contrast, some proteins increase to play their specific functions, such as RBM3 (Zhu et al. 2016a). RBM3 participates in many physiological activities such as transcription and translation, microRNA biogenesis, circadian rhythm, neuroprotection, apoptosis, cell cycle, cell stem, cancer, immunity, and virus infection (Danno et al. 2000; Sureban et al. 2008; Jögi et al. 2009). RBM3, as a stress response protein, regulates a variety of cell physiological activities under various stress conditions, such as cold stress, endoplasmic reticulum stress, hypoxia, and radiation (Lebsack et al. 2010; Zhu et al. 2016b). However, RBM3 is widely considered a cold shock protein because of its sensitive and rapid response to cold stress (Xia et al. 2018). RBM3 has an excellent cytoprotective effect under hypothermia, and even the upregulation of RBM3 in neurons is irreplaceable as a potential effector for neuroprotective effects induced by mild hypothermia in the clinic (Peretti et al. 2015). RBM3 reduces cell apoptosis from endoplasmic reticulum stress (Zhu et al. 2016b). RBM3 has certain modulation on cell death induced by polyglutamine (Wellmann et al., 2010). In addition, it has been reported that RBM3 has a significant function in anti-apoptosis (Ma et al. 2018). RBM3 is also considered a key regulator in maintaining quality and ameliorating the atrophy of skeletal muscle (Dupont-Versteegden et al. 2008; Van Pelt et al. 2018). RBM3 increases phosphorylation of AKT at mild hypothermia, and AKT signaling regulates nutrient absorption and metabolism through multiple downstream targets (Liu et al. 2021a, b, c). Some studies have demonstrated that the upregulation of RBM3 expression at mild hypothermia is regulated by p65 activity and phosphorylation (Ushio and Eto 2018). The cytoprotective mechanism of RBM3 is unclear and its rapid upregulation in response to cold remains to be clarified.
O-GlcNAcylation is an atypical, reversible, and dynamic glycosylation modification, which is precisely maintained by the synergy of OGT and OGA (Yang and Qian 2017). OGT binds a single N-acetylglucosamine (GlcNAc) provided only by UDP-N-acetylglucosamine to serine and threonine residues of the target protein (Parker et al. 2021). In contrast, OGA hydrolyzes GlcNAc of O-GlcNAcylated protein (Martinez-Fleites et al. 2008). O-GlcNAcylation rapidly responds to various physiological signals and environmental conditions, thus controlling the activity and function of the target protein (Fisi et al. 2017; Balana et al. 2021). This leads to O-GlcNAcylation as a “stress receptor” (Akan et al. 2018). Glucose generates UDP-N-acetylglucosamine through the hexosamine biosynthesis pathway for O-GlcNAcylation, which is connected in a series with the metabolism of glucose, lipid, amino acid, and nucleotide (Chatham et al. 2021). Therefore, O-GlcNAcylation combines the systemic metabolic state with the cellular regulation of signal transduction, transcription, and protein degradation as a “nutrition sensor” (Liu et al. 2021b). In addition, the overall O-GlcNAcylation increased and enhanced glycolysis in response to cold stress (Yao et al. 2018). As an emerging regulator of skeletal muscle, O-GlcNAcylation participates in many physiological processes of skeletal muscle, such as glucose metabolism and insulin resistance (Wang et al. 2016; Lambert et al. 2018; Shi et al. 2018). OGT-mediated O-GlcNAcylation promotes p65 activation to regulate the expression of downstream proteins (Zhang et al. 2015).
Therefore, we speculate that that RBM3 plays a protective role by enhancing AKT phosphorylation to increase glucose metabolism and reduce apoptosis in skeletal muscle of mice under acute cold exposure, while RBM3 is upregulated by the O-GlcNAcylation of p65 mediated by OGT.
Materials and methods
Animal and acute cold exposure
The experimental animals were male C57BL/6 mice aged 8 weeks old and weighing 25–28 g. Mice were randomly divided into three groups: control, 2 h, and 4 h acute cold exposure groups (eight mice per group). Each 4 mice were placed in standard polystyrene cages. Each cage contained 200 g of soft sawdust bedding. Feeding temperature was set at 28 ± 0.5 °C, humidity was 40 ± 5%, illumination intensity was 200 lx, and the light–dark cycle ratio was 12/12. Mice were free to drink water but food intake was limited (from 8:00 p.m. to 8:00 a.m.). At the end of adaptive feeding, the cold exposure groups were exposed to 4 °C for 2 and 4 h. The control group remained at 28 °C. The mice were anesthetized with ether and euthanized by cervical dislocation. Blood and gastrocnemius were collected after the mice were anesthetized and then euthanized.
In a second set of experiments, mice were male C57BL/6 mice weighing 25–28 g and aged 8 weeks, which were randomly divided into the control cold exposure group, DMSO-injected cold exposure group, and wortmannin (a classical AKT inhibitor)-injected cold exposure group (five mice in each group). Wortmannin was intraperitoneally injected into mice at a dose of 0.5 mg/kg according to their body weight. One hour after injection, the three groups of mice were exposed to 4 °C for 4 h at the same time. Feeding and treatment were strictly consistent. The sample collection procedure is the same as above.
Muscle-specific Ogt-deficient C57BL/6 mice (KO for short) were purchased from the Jackson Laboratory. Male KO mice aged 8 weeks and ten wild-type mice (WT for short) in the same nest were divided into a normal temperature control group and a 4-h cold exposure group (5 mice in each group). The 4-h cold exposure group was exposed to 4 °C for 4 h. Feeding and treatment were strictly consistent. The sample collection procedure is the same as above.
Determination of biochemical indices
Serum glucose level was measured using a biochemical analyzer (IDEXX Laboratories, USA). Serum insulin and glucagon levels were measured using an ELISA kit (#CEA448Mu and CEB266Mu, Cloud-Clone Corp., USA). Fructose-1,6-diphosphate (FDP) and pyruvic acid (PA) contents in skeletal muscle were measured by assay kits (#BC2240 and BC2200, Solarbio, China). The above operations are carried out in full accordance with their instructions.
Determination of adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) in skeletal muscle of mice
Fresh skeletal muscle tissue with a mass of 100 mg was taken and put into a glass tissue homogenizer, and 1 mL of 0.5 mol/L of precooled HClO4 solution was added and homogenized on ice. The tissue homogenate obtained was transferred to another centrifuge tube and then centrifuged. The supernatant was transferred to a new centrifuge tube. The same volume of 1 mol/L K2HPO4 was added and the pH was adjusted to neutral after mixing. After the second centrifugation, the supernatant was filtered by a disposable needle filter (0.45 µm). Finally, ATP, ADP, and AMP were determined by HPLC.
Periodic acid Schiff (PAS) staining
Fresh skeletal muscle samples were fixed in 4% paraformaldehyde. The samples were routinely dehydrated and embedded in paraffin, and 5 µm thick sections were prepared. The sections were stained by the glycogen periodic acid Schiff stain kit (#G1281, Solarbio, China) for examining the glycogen content of gastrocnemius tissues.
Western blot analysis
An equivalent amount of protein from each group was separated by SDS-PAGE, then electrotransferred to a PVDF membrane (#IPVH00010, Millipore, Germany). The PVDF membrane was immersed in 10 mL of Ponceau staining solution and shaken for 5 min. After the clear band appeared, each entire band was preserved by a Chemidoc XRS Gel Imaging System and analyzed by ImageJ software. Subsequently, the membranes were sealed in the blocking buffer for 1 h at ordinary temperature and incubated with the primary antibodies overnight at 4 °C with shaking. This was followed by incubation with the secondary antibody for 1 h at ordinary temperature. Then, the membranes were visualized by chemiluminescence detection using Luminata Crescendo Western HRP substrate (WBLUR0100, Millipore, Germany). Blots were preserved by the Chemidoc XRS Gel Imaging System and analyzed by ImageJ software.
Antibodies used were as follows: AKT (#9272, 1:1000), phospho-AKTSer473 (#4060, 1:1000), phospho-AKTThr308 (#13,038, 1:1000), phospho-glycogen synthase kinase 3β (GSK3β)Ser9 (#9323, 1:1000), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2) (#13,045, 1:1000), phospho-PFKFB2Ser483 (#13,064, 1:1000), glycogen synthase (GS) (#3886, 1:1000), phospho-GSSer641 (#3891, 1:1000), phospho-p65Ser536 (#3033, 1:1000), phospho-insulin receptor substrate (IRS)Ser302 (#2384, 1:1000), and OGT (#24,083, 1:1000) were purchased from Cell Signaling Technology (USA); RBM3 (#ab134946, 1:500), glucose transporter 4 (Glut4) (#ab654, 1:2000), GSK3β (#ab32391, 1:2000), and O-GlcNAc (#ab93858, 1:1000) were purchased from Abcam (UK); and p65 (#10,745–1-AP, 1:1000), IRS (#17,509–1-AP, 1:1000), Cleaved Caspase-3 (#66,470–2, 1:500), B-cell lymphoma-2 (Bcl-2) (#60,178–1-lg, 1:2000), and Bcl-2 associated X protein (Bax) (#60,267–1-lg, 1:2000) were purchased from Proteintech (USA). Goat anti-rabbit IgG (#SA00001-2, 1:20,000) and goat anti-mouse IgG (#SA00001-1, 1:20,000) were also purchased from Proteintech.
Cell culture, RBM3 overexpression, and mild hypothermia treatment
The mouse myoblast cell line (C2C12) was cultured in DMEM (#C11995500BT, Gibco, USA) containing 10% fetal bovine serum (#10,099, Gibco) and 100 U/mL penicillin and streptomycin (#P1400, Solarbio, China) in a cell incubator at 37 °C with 5% CO2.
The RBM3 overexpression lentivirus was from Beijing Hesheng Gene Technology Co., Ltd (Beijing, China). The virus was diluted with the appropriate multiplicity of infection (120:1) and mixed with a polybrene transfection enhancer (#H8761, Solarbio, China). The final polybrene concentration was 6 µg/mL, and the mixture was added to each well. The efficiency of lentivirus infection was detected by fluorescence microscopy 72 h later. Control cells, empty vector virus-infected cells, and RBM3 overexpressed cells were cultivated in a cell incubator at 32 °C for 4 h.
Validation of O-GlcNAcylation of p65
Succinylated wheat germ agglutinin (sWGA) has a strong specificity for GlcNAc. Therefore, it can identify the GlcNAc linked on proteins to detect O-GlcNAcylated proteins. sWGA-agarose beads were added to 500 µg of total protein from each group and incubated for 12 h at 4 °C. The supernatant was discarded after centrifugation. The beads were washed three times (5 min each time) with lysis buffer. After the liquid was air-dried, the loading buffer was added and boiled for 10 min for denaturation. The supernatant was centrifuged for subsequent western blotting.
Monitoring of p65 signaling activity
C2C12 cells were transfected with a p65 reporter plasmid (#D2206, Beyotime, China) using Lipo8000™ transfection reagent (#DC0533, Beyotime, China). For more information and operation of this plasmid and transfection, please refer to the instructions. After 48 h, the cells were divided into four groups: control, 4 h cold, 4 h cold + alloxan (an OGT inhibitor), and 4 h cold + thiamet G (an OGA inhibitor). Alloxan and thiamet G were added to 4 h cold + alloxan group and 4 h cold + thiamet G group, respectively, for 6 h. All cold groups were hypothermic for 4 h at 32 °C. The final concentration of alloxan was 50 µmol/mL, while the final concentration of thiamet G was 20 µmol/mL. Cells of each group were collected and lysed. The activity of p65 were detected using a Dual-Lumi II luciferase reporter gene assay kit (#RG089S, Beyotime, China) with a chemiluminescence immunoassay analyzer. The relevant operation and calculation completely follow the instructions of the kit.
Statistical analysis
All data are shown as the mean ± SEM. All data were analyzed by GraphPad Prism. Comparisons between groups were performed by one-way or two-way ANOVA followed by Tukey’s post-hoc test. Statistically significant differences are indicated as * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Results
Acute cold exposure increases energy expenditure in mice
Serum glucose levels continued to decrease significantly with prolonged acute cold exposure (P < 0.05, Fig. 1A). Insulin and glucagon are a pair of important antagonistic hormones that regulate blood glucose levels. Serum insulin levels fluctuated at 2 h of acute cold exposure and subsequently recovered (P < 0.01, Fig. 1B). Acute cold exposure resulted in an increase of glucagon levels at 4 h (P < 0.001, Fig. 1C). Glycogen in skeletal muscle accounts for about two-thirds of the total glycogen in the whole body and is the most important form of energy storage in skeletal muscle. Changes in their ratios also indicate an increase in energy mobilization and suppression of energy reserves in mice under acute cold exposure (Fig. 1D). The glycogen of skeletal muscle was decreased by acute cold exposure in a time-dependent manner (Fig. 1E and F), indicating that glycogen depletion of mouse skeletal muscle was caused by acute cold exposure. FDP (glycolytic intermediate) increased significantly at 2 h of acute cold exposure (Fig. 1G), as did PA (glycolytic end product) (Fig. 1H). In addition, the level of ATP/(ADP + AMP) in skeletal muscle was increased after acute cold exposure (Fig. 1L). These results suggest that acute cold exposure increases systemic energy mobilization and expenditure, which promotes increased skeletal muscle glycolysis to meet the high levels of energy requirements.
Fig. 1.
Acute cold exposure increased energy expenditure in skeletal muscle of mice. Simple acute cold exposure with different intensities at 4 °C was applied to C57BL/6 mice, and blood and skeletal muscle samples were collected from each group. Effect of acute cold exposure on serum glucose (A), insulin (B), glucagon (C), and insulin/glucagon molar ratio (D) in each group of mice. Effects of acute cold exposure on the glycogen level (E and F) of skeletal muscle in each group of mice. Effect of acute cold exposure on the FDP (G) and PA (H) levels in the skeletal muscles of mice. Effect of acute cold exposure on ATP (I), ADP (J), AMP (K), and ATP/(ADP + AMP) (L) in the skeletal muscles of mice. The data are presented as the mean ± SEM (n = 8). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001
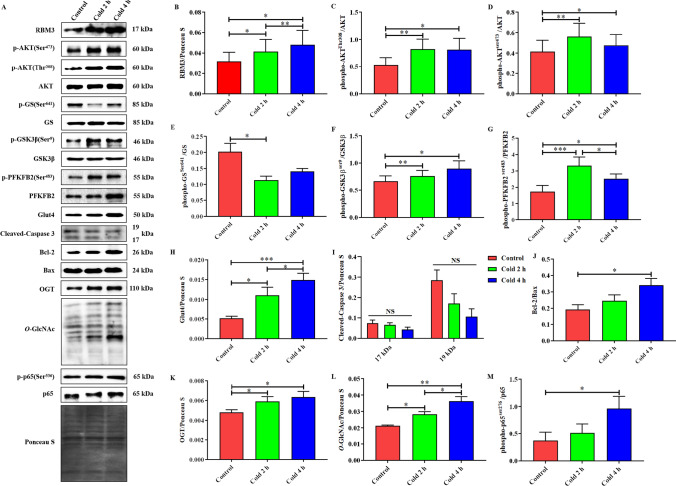
Acute cold exposure upregulated RBM3, activated AKT, enhanced glucose metabolism, and reduced apoptosis in the skeletal muscle of mice.
RBM3 was upregulated in a time-dependent manner under acute cold exposure (Fig. 2B). Subsequently, acute cold exposure increased AKT phosphorylation at Ser473 and Thr308 (Fig. 2C and D). Glut4 and the phosphorylation of GSK3β and PFKFB2 were enhanced (Fig. 2F-H), but GS phosphorylation was inhibited by acute cold exposure (Fig. 2E). Cleaved caspase-3 did not change (Fig. 2I), while Bcl-2/Bax significantly increased (Fig. 2J). Notably, OGT and its mediated O-GlcNAcylation levels increased in a time-dependent manner and p65 phosphorylation also showed similar changes under acute cold exposure.
Fig. 2.
Acute cold exposure upregulated RBM3, activated AKT, enhanced glucose metabolism, and induced apoptosis in skeletal muscle of mice. Simple acute cold exposure with different intensities at 4 °C was applied to C57BL/6 mice, and skeletal muscle samples were collected from each group. Western blot analysis of RBM3, glucose metabolism, and apoptosis-related proteins in mouse skeletal muscle after acute cold exposure is shown in A. The protein expression levels and phosphorylation status of RBM3 (B), AKT (C and D), GS (E), GSK3β (F), PFKFB2 (G), Glut4 (H), Cleaved-Caspase3 (I), Bcl-2/Bax (J), OGT (K), O-GlcNAc (L), and p65 (M) in the skeletal muscles of mice. The data are presented as the mean ± SEM (n = 8). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, and *** P < 0.001
Based on the above results and related literature, we hypothesized that the upregulation of RBM3 mediated by acute cold exposure may increase AKT phosphorylation to enhance glycolysis and reduce apoptosis, which may be achieved by the OGT-mediated O-GlcNAcylation of p65.
Overexpression of RBM3 enhanced the phosphorylation of AKTat Ser473 and Thr308 inC2C12 cells under mild hypothermia
We overexpressed RBM3 successfully in vitro. RBM3 increased over time in all groups under mild hypothermia at 32 °C. RBM3 was significantly increased when C2C12 cells were infected by RBM3 lentivirus (Fig. 3B and C). The phosphorylation of AKT at Ser473 and Thr308 was enhanced by mild hypothermia. More importantly, overexpression of RBM3 significantly increased the phosphorylation of AKT at Ser473 and Thr308 (Fig. 3B, D, and E). These results further confirmed that RBM3 promoted the phosphorylation of AKT.
Fig. 3.
RBM3 overexpression and its enhanced phosphorylation of AKT at Ser473 and Thr308 in C2C12 cells under mild hypothermia. In vitro, C2C12 cells infected with RBM3 lentivirus for 72 h at 37 °C (A), followed by a mild hypothermia treatment for 4 h at 32 °C. Western blot analysis of RBM3 and AKT expression and phosphorylation in each group (B). Effects of mild hypothermia and lentivirus infection on RBM3 (C) expression in C2C12 cells. Effects of RBM3 overexpression on the phosphorylation of AKT at Ser473 (D) and Thr.308 (E) in C2C12 cells. The data are presented as the mean ± SEM (n = 6). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, and *** P < 0.001
AKT and its phosphorylation are essential for promoting glucose metabolism and the cytoprotection of RBM3 in skeletal muscle of mice under acute cold exposure
The AKT phosphorylation was significantly decreased after the injection of wortmannin (Fig. 4B and C), which reduced the levels of GS, GSK3β, PFKFB2, and their phosphorylation (Fig. 4D–F). Bcl-2/Bax was significantly decreased and Caspase-3 was significantly increased after wortmannin injection (Fig. 4G and H). In addition, p65 phosphorylation also showed similar changes (Fig. 4I). This is an interesting result, suggesting that AKT may have some feedback regulation on p65. IRS-1 phosphorylation has not changed (Fig. 4J).
Fig. 4.
AKT and its phosphorylation are essential for promoting glucose metabolism and cytoprotection of RBM3 in skeletal muscle of mice under acute cold exposure. Inhibition of AKT activation using wortmannin in vivoto explore its role in RBM3 cytoprotection under acute cold exposure. Western blot analysis of related proteins in mouse skeletal muscle with wortmannin injection under acute cold exposure (A). Effects of wortmannin on protein expression levels and phosphorylation of AKT (B and C), GS (D), GSK3β (E), PFKFB2 (F), Bcl-2/Bax (G), Cleaved-Caspase3 (H), p65 (I), and OGT (J) in skeletal muscle of mice under acute cold exposure. The data are presented as the mean ± SEM (n = 5). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, and *** P < 0.001
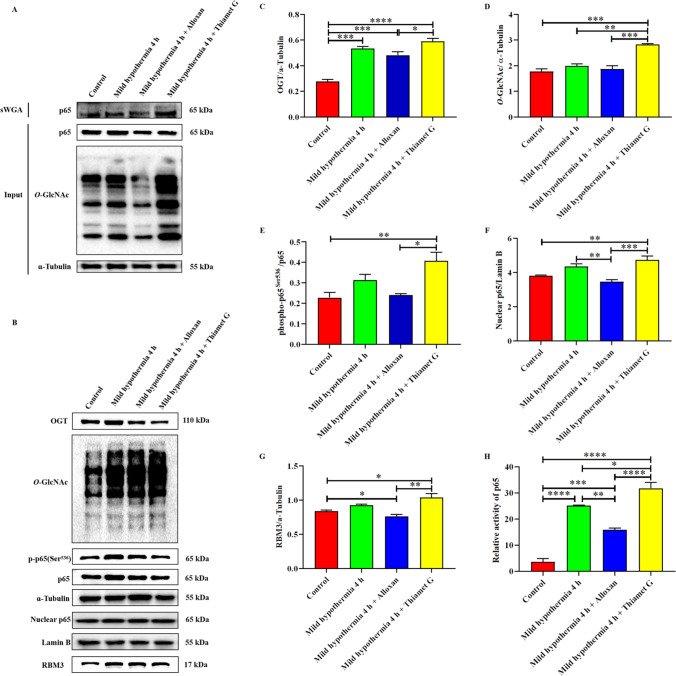
O-GlcNAcylation of p65 is mediated by OGT to increase nuclear translocation and activity, upregulating RBM3 in C2C12 cells under mild hypothermia
We demonstrated that OGT mediates O-GlcNAcylated p65 in vitro under mild hypothermia using a sWGA method for the first time. The western blot analysis of sWGA is shown in Fig. 5A. We also found that an increase of O-GlcNAcylation can promote the phosphorylation of p65 at Ser536, and vice versa (Fig. 5B–E). Subsequently, we isolated the nuclear protein and examined the changes in nuclear p65. We found that increased O-GlcNAcylation leads to an increase of nuclear p65, suggesting that O-GlcNAcylation promotes the nuclear translocation of p65 (Fig. 5F). Monitoring results of p65 signal activity showed that O-GlcNAcylation had a positive effect on p65 activity (Fig. 5F). Finally, the expression of RBM3 changed as expected (Fig. 5G). Based on the above results, it was confirmed that OGT-mediated O-GlcNAcylation enhanced the nuclear translocation and activity of p65 to promote RBM3 expression under mild hypothermia, which was also consistent with the role of O-GlcNAcylation as a stress receptor.
Fig. 5.
O-GlcNAcylation of p65 mediated by OGT increases its nuclear translocation and activity, thereby upregulating RBM3 in C2C12 cells under mild hypothermia. Global O-GlcNAcylation levels were regulated by Alloxan (an OGT inhibitor) and Thiamet G (an OGA inhibitor) in C2C12 cells to explore its effects on p65 and RBM3. Western blot analysis of O-GlcNAcylated p65 detected by sWGA (A). Western blot analysis of related proteins in C2C12 cells under mild hypothermia (B). Effects of Alloxan and Thiamet G on O-GlcNAcylation (D) and OGT in C2C12 under mild hypothermia. Effects of overall O-GlcNAcylation level on the phosphorylation and nuclear translocation of p65 (E and F) and RBM3 (G) in C2C12 cells under mild hypothermia. p65 relative activity of each group determined by dual-luciferase assay (H). The data are presented as the mean ± SEM (n = 6). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001
RBM3 upregulated by O-GlcNAcylation of p65 promotes AKT phosphorylation to enhance glucose metabolism and reduce apoptosis of skeletal muscles in mice during acute cold exposure
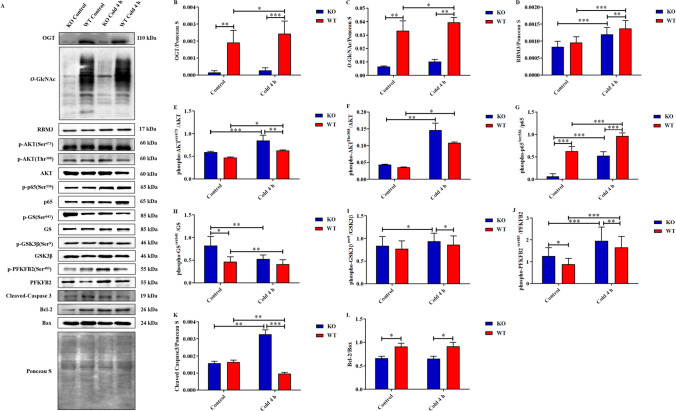
To further verify our conjecture, we first constructed skeletal muscle Ogt-knockout C57BL/6 mice using a Cre-LoxP conditional gene editing system (Fig. 6A). The expression of OGT in the skeletal muscle of KO mice was significantly decreased in both the control and cold exposure groups (Fig. 7A and B). This was followed by an extremely significant decline in O-GlcNAcylation levels (Fig. 7A and C), indicating that Ogt was successfully knocked out in the skeletal muscle of KO mice. OGT in the 4-h cold WT group was significantly increased, whereas OGT in the 4-h cold KO group was also increased but not significantly. A similar change occurred in O-GlcNAcylation (Fig. 7A–C).
Fig. 6.
Effects of OGT deficiency on body temperature, serum glucose, serum insulin, skeletal muscle FDP, and PA in mice under acute cold exposure. Muscle-specific Ogt-deficient C57BL/6 mice were constructed to further verify in vivo that O-GlcNAcylation of p65 upregulates RBM3 under acute cold exposure. Breeding strategy used to generate wild-type (WT) control mice and skeletal muscle-specific Ogt-knockout (KO) mice (A). Changes of body temperature (B), serum glucose (C), serum insulin (D), skeletal muscle FDP (E), and PA (F) in mice induced by OGT deficiency under acute cold exposure. The data are presented as the mean ± SEM (n = 5). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001
Fig. 7.
RBM3 upregulated by O-GlcNAcylation of p65 promotes AKT phosphorylation to enhance glucose metabolism and reduce apoptosis of skeletal muscles in mice during acute cold exposure. Simple acute cold exposure for 4 h at 4 °C was applied to KO and WT mice, and skeletal muscle samples were collected from each group. Western blot analysis of OGT, RBM3, glucose metabolism, and apoptosis-related proteins in the skeletal muscles of each group (A). The protein expression levels and phosphorylation status of OGT (B), O-GlcNAc (C), RBM3 (D), AKT (E and F), p65 (G), GS (H), GSK3β (I), PFKFB2 (J), Cleaved-Caspase3 (K), and Bcl-2/Bax (L) in the skeletal muscles of each group. The data are presented as the mean ± SEM (n = 5). Statistically significant differences are indicated: * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001
We first measured the core body temperature of mice in each group and found that the body temperature in KO mice was lower than WT mice (Fig. 6B). This is a very interesting result, suggesting that OGT may contribute to thermogenesis and thermoregulation under acute cold exposure. The results showed that cold exposure significantly reduced the blood glucose level, and the blood glucose level of KO mice was significantly lower than that of WT mice (Fig. 6C). The results showed that there were similar changes in the insulin levels of each group during acute cold exposure, suggesting that OGT deletion may lead to insulin resistance (Fig. 6D).
The expression of RBM3 in skeletal muscle of KO mice was lower than that of WT mice, especially under acute cold exposure (Fig. 7D). Phosphorylation of AKT at Ser473 and Thr308 in skeletal muscle of KO mice was higher than that of WT mice (Fig. 7E and F). The effect of acute cold exposure and RBM3 on AKT phosphorylation was consistent with our hypothesis, but the increase in AKT phosphorylation caused by OGT deficiency was beyond our expectations, which aroused our interest. The phosphorylation of p65 at Ser536 in skeletal muscle of KO mice is lower than that of WT mice, which still supports our hypothesis. We think this interesting result is because OGT deficiency causes a dramatic decrease in O-GlcNAcylation, thus liberating the interaction with phosphorylation in AKT. The phosphorylation levels of GS, GSK3β, and PFKFB2 were enhanced in the skeletal muscle of KO mice (Fig. 7H–J). Similarly, the increased content of glycolytic intermediates and end products in skeletal muscle of KO mice also suggests an increased glycolytic flux (Fig. 6E and F). The ratio of Bcl-2 to Bax and the expression of Caspase3 in skeletal muscle of KO mice were significantly higher than those of WT mice (Fig. 7K and L). These results suggest that the apoptosis of skeletal muscle in KO mice is increased and OGT has a certain level of cytoprotection.
Discussion
A series of systemic metabolic changes ensued to maintain core body temperature in a cold environment through cold-induced thermogenesis (Blondin et al. 2015; Labbé et al. 2015). Acute cold exposure increases peripheral tissue energy utilization and liver glycolysis and gluconeogenesis, thus satisfying increases in energy expenditure and contributing to blood glucose homeostasis, which is critical for ensuring an energy supply to tissues (Alvim et al. 2015; Wang et al. 2020). Our serum glucose test results showed that the serum glucose level continued to decline, which suggests that acute cold exposure affected the changes of energy metabolism in peripheral tissues and increased the utilization and uptake of glucose. The cooperation of insulin and glucagon regulates the fluctuation of blood glucose levels over a small range under acute cold exposure (Wang and Wahl 2014). Serum insulin levels increased at the initial stage of acute cold exposure and then recovered. Insulin is secreted dynamically according to compensatory nutritional and metabolic changes, which maintain glucose homeostasis. The increase in the insulin level may be caused by rapid energy mobilization during acute cold exposure. Serum glucagon levels were relatively steady at the initial stage of acute cold exposure and then increased. Glucagon promotes gluconeogenesis and glycogen decomposition to satisfy energy demands in case of malnutrition. This resulted in the increase of serum glucagon with the extension of acute cold exposure time.
Our results show that the phosphorylation of PFKFB2 at Ser483 was enhanced, indicating that acute cold exposure enhanced the glycolytic activity in mouse skeletal muscle. FDP is a metabolic intermediate product of glycolysis, which ensures adequate ATP (Zhang et al. 2017). The increase of FDP and PA (the product of glycolysis) levels more directly indicates that acute cold exposure increased glycolysis in the skeletal muscle of mice. This is also one of the main reasons why the significant increase trend of ATP/(ADP + AMP) is consistent with that of FDP and PFKFB2. PFKFB2 and its regulation of glycolysis are AKT-dependent (Novellasdemunt et al. 2013; Rodríguez-García et al. 2017). We also observed increased phosphorylation of AKT at Ser473 and Thr308. AKT activation promotes the phosphorylation and inactivation of GSK3β, which in turn will further cause an increase in the activity and abundance of GS and a decrease in the phosphorylation of GS and ultimately upregulates glycogen synthesis (Frame and Cohen 2001; Li et al. 2019b). The phosphorylation level of GSK3β at Ser9 was increased and the phosphorylation level of GS at Ser461 was decreased, indicating that glycogen synthesis was activated by acute cold exposure. In addition, glucose transport in skeletal muscle largely depends on Glut4 (Richter and Hargreaves 2013). Our study showed that Glut4 expression increased, which further explained how acute cold exposure increased glucose uptake and transport in mouse skeletal muscle.
RBM3, a highly efficient cold shock protein, is sensitive to hypothermia and is rapidly upregulated in response to cold stress and reduces stress damage, but is inactivated by heat (Jackson et al. 2015; Knott 2015). Inhibition of overall protein synthesis is caused by hypothermia, which is one of the main causes of body damage (Rathjen et al. 2019). However, RBM3 is completely contrary to this inhibition and even regulates the expression of some proteins at the translation level by binding to different transcripts, so that cells respond quickly to environmental signals and quickly adapt to the harsh environment (Al-Astal et al. 2016). Our previous study has confirmed that the upregulation of RBM3 induced by acute cold exposure in the liver promotes survival (Shi et al. 2019). Similarly, acute cold exposure upregulated RBM3, which reduced apoptosis in the skeletal muscle of mice. There is a lot of evidence that RBM3 upregulation caused by acute cold exposure plays a positive cytoprotective role (Chip et al. 2011; Peretti et al. 2015; Xia et al. 2018). RBM3 reduces radiation-induced apoptosis through PI3K/AKT/Bcl-2 (Ma et al. 2018). RBM3 affects p38, JNK, and the Bcl-2 family to reduce apoptosis under UV irradiation (Zhuang et al. 2017). RBM3 alleviated NO-induced apoptosis by inhibiting the p38 signal to regulate miR-143 (Yang et al. 2017). Overexpression of RBM3 significantly reduced necrosis and apoptosis to promote survival in C2C12 cells under mild hypothermia (Ferry et al. 2011). In addition, RBM3 overexpression also reduced skeletal muscle atrophy in rats as a skeletal muscle mass regulator (Dupont-Versteegden et al. 2008). We also found that Cleaved Caspase-3 levels did not change and that the Bcl-2/Bax ratio increased under acute cold exposure. Hence, these results suggest that acute cold exposure-induced upregulation of CIRP alleviates apoptosis in mouse skeletal muscle.
Based on these results, we speculate that the upregulation of RBM3 by mild hypothermia enhances glucose metabolism and reduces apoptosis through promoting the phosphorylation of AKT in mouse skeletal muscle. We constructed RBM3 overexpression in vitro to clarify the impact of RBM3 on AKT. Subsequently, we found that RBM3 overexpression significantly enhanced the phosphorylation of AKT at Ser473 and Thr308 in C2C12 cells at 37 and 32 °C, which indicates that RBM3 activates AKT. The PI3K/AKT signaling pathway regulates nutrition and metabolism through a variety of downstream targets (Cui et al. 2018; Hoxhaj and Manning 2020). The IRS/PI3K/AKT pathway contributes to cell survival by enhancing glycolysis to maintain glucose homeostasis during stress (Schultze et al. 2012; Huy et al. 2018; Abdel-Wahab et al. 2019). AKT is indispensable as a mediator to inhibit apoptosis and autophagy for cell survival (Benbrook and Masamha 2011; Yang et al. 2018). AKT protects cells from apoptosis by blocking the mitochondrial release of CYT-C and inactivating FoxOs (Fulda 2014; Goldbraikh et al. 2020). The phosphorylation of AKT at Ser473 inactivates Bad and Caspase-9 (Li et al. 2012). AKT increases Bcl-2/Bax and reduces Caspase3 by weakening apoptosis induced by TNF-α (Li et al. 2019a). Our results also showed that wortmannin significantly inhibited the phosphorylation of AKT, which led to the weakening of glucose metabolism and the increase of apoptosis. This further indicates that AKT and its phosphorylation are a regulatory center controlling glucose metabolism and apoptosis.
OGT activity and overall O-GlcNAcylation level are regulated by various stress stimuli, thus participating in many cell processes to increase survival (Lee et al. 2016; Han et al. 2017). OGT is essential for energy homeostasis, systemic insulin sensitivity, and mitochondrial biogenesis (Shi et al. 2018). O-GlcNAcylation promotes cold-induced thermogenesis through mitochondrial biogenesis in brown adipose tissue (Ohashi et al. 2017). This may be the reason that core body temperature and blood glucose were lower in KO mice. On the other hand, it is also possible that the defect of OGT leads to the damage of heat preservation. Evidence shows that OGT plays a key role in the deposition of fat, especially visceral fat (Yang et al. 2020). Hypothermia caused by a drop in core body temperature results in a vicious cycle of dysregulation of metabolic homeostasis and disruption of certain physiological systems such as altered microcirculation, hypoxia, and ROS production, ultimately leading to apoptosis and/or necrosis (Castellani and Young 2016). Transcription and translation of cold shock proteins such as RBM3, cold-inducible RNA-binding proteins, and heat shock proteins are activated under these adverse conditions and exert their cytoprotective effects (Shi et al. 2019). Our previous studies found that when OGT and overall O-GlcNAcylation are increased by acute cold exposure, they have certain cytoprotective effects in the liver (Yao et al. 2018; Liu et al. 2021a). Consistent with this, OGT and overall O-GlcNAcylation were also increased in mouse skeletal muscle under acute cold exposure. We found a very interesting phenomenon, changes in the trends of OGT, O-GlcNAcylation, p65 phosphorylation, and RBM3 are highly consistent, which encourages us to explore their potential relationship. Several studies have shown that OGT may mediate the O-GlcNAcylation of p65 under certain conditions (Xing et al. 2011; Hu et al. 2022). However, our study has discovered that O-GlcNAcylated p65 exists in the skeletal muscle of mice under acute cold exposure. The O-GlcNAcylation of p65 inhibits the interaction between NF-κB and IκB and promotes the nuclear translocation and transcriptional activation of p65 (Ma et al. 2013). In our study, the O-GlcNAcylation of p65 enhanced its activity and nuclear translocation in C2C12 cells during mild hypothermia and contributes to the upregulation of RBM3. We confirmed that OGT deletion reduced the rapid response of RBM3 in skeletal muscle of KO mice under acute cold exposure. However, the decrease of RBM3 in skeletal muscle of KO mice was not as great as expected. This may be because RBM3 has compensatory pathways that are not dependent on OGT. There is an internal ribosome entry site in the 5′precursor sequence of RBM3 mRNA, which could recruit ribosomes to enhance its expression (Matsuda et al. 2011). This is similar to the mild cold-responsive element found in the upstream sequence of the CIRP promoter to enhance translation, for rapid expression in response to cold exposure (Sumitomo et al. 2012). In addition, it was reported that FAK/Src signaling upregulates RBM3 to play a cytoprotective role under mild hypothermia (Yuan et al. 2021). Meanwhile, HIF-1, TrkB, and MZF1 also promote RBM3 transcription under mild hypothermia (Wellmann et al. 2004; Peretti et al. 2021; Zhang et al. 2021). These are the reason why the expression of RMB3 decreased but not completely decreased in KO mice. Therefore, it should be noted that the regulation of RBM3 expression is not entirely dependent on OGT-mediated O-GlcNAcylation of p65. O-GlcNAcylation is a key regulator of glycolysis, and an OGT defect relieves the O-GlcNAcylation restriction on glycolysis (Bacigalupa et al. 2018). PI3K/AKT regulates the phosphorylation of NF-κB (Lee et al. 2003; Liu et al. 2019). The elimination of PI3K/AKT-mediated glycolysis flux limitation by the nutritional and stress sensor effects of O-GlcNAcylation due to OGT deficiency explains the abnormal increase of AKT phosphorylation and glucose metabolism in KO mice. Our results also confirm this and these changes are consistent with the above increases in AKT phosphorylation. These results suggest that AKT may be involved in the interaction between dynamic phosphorylation and O-GlcNAcylation of p65 to form a negative feedback regulation. This is interesting and will be investigated in our next research direction.
Conclusion
In summary, O-GlcNAcylation of p65 upregulates RBM3 to promote AKT phosphorylation to enhance glucose metabolism and reduce apoptosis in skeletal muscle under acute cold exposure. A potential physiological mechanism of RBM3 function in mouse skeletal muscle under acute cold exposure is proposed in Fig. 8.
Fig. 8.
The potential physiological mechanism of RBM3 in skeletal muscle of mice under acute cold exposure
Acknowledgements
Thanks to Professor Hongming LV, Bin Xu, and Professor Shuai Lian for their guidance in the manuscript writing process.
Author contribution
Yang Liu designed this study and compiled the manuscript. Hongzhao Shi analyzed the data. Yajie Hu perfected the experimental design. Ruizhi Yao and Peng Liu performed the literature search. Yuying Yang provided technical support during the experiments. Shize Li supervised and evaluated all the work. The final manuscript was read and approved by all authors.
Funding
This study was funded by the General Project of National Natural Science Foundation of China (31772695), Natural Science Foundation of Heilongjiang Province (ZD2019C004), and Graduate Innovative Research Project of Heilongjiang Bayi Agricultural University (YJSCX2021-Z01).
Data availability
The datasets used during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
Animal Protection and Utilization Committee of Heilongjiang Bayi Agricultural University (Daqing, China) approved all experiments. All animal care procedures were performed in accordance with Chinese Guidelines for Animal Welfare. All operations and efforts were aimed at reducing the pain and number of animals.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Liu and Hongzhao Shi contributed equally to this work.
References
- Abdel-Wahab AF, Mahmoud W, Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. 2019;150:104511. doi: 10.1016/j.phrs.2019.104511. [DOI] [PubMed] [Google Scholar]
- Akan I, Olivier-Van Stichelen S, Bond MR, Hanover JA. Nutrient-driven O-GlcNAc in proteostasis and neurodegeneration. J Neurochem. 2018;144:7–34. doi: 10.1111/jnc.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Astal HI, Massad M, AlMatar M, Ekal H. Cellular functions of RNA-binding motif protein 3 (RBM3): clues in hypothermia, cancer biology and apoptosis. Protein Pept Lett. 2016;23:828–835. doi: 10.2174/0929866523666160628090340. [DOI] [PubMed] [Google Scholar]
- Alvim RO, Cheuhen MR, Machado SR, Sousa AG, Santos PC. General aspects of muscle glucose uptake. Anais Da Academia Brasileira De Ciencias. 2015;87:351–368. doi: 10.1590/0001-3765201520140225. [DOI] [PubMed] [Google Scholar]
- Bacigalupa ZA, Bhadiadra CH, Reginato MJ. O-GlcNAcylation: key regulator of glycolytic pathways. J Bioenerg Biomembr. 2018;50:189–198. doi: 10.1007/s10863-018-9742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balana AT, et al. O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity. Nat Chem. 2021;13:441–450. doi: 10.1038/s41557-021-00648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook DM, Masamha CP. The pro-survival function of Akt kinase can be overridden or altered to contribute to induction of apoptosis. Curr Cancer Drug Targets. 2011;11:586–599. doi: 10.2174/156800911795655994. [DOI] [PubMed] [Google Scholar]
- Blondin DP, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani JW, Young AJ. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196:63–74. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Zhang J, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol Rev. 2021;101:427–493. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chip S, et al. The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis. 2011;43:388–396. doi: 10.1016/j.nbd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Cui X et al (2018) Scutellariae radix and Coptidis Rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol Sci 19(11):3634 [DOI] [PMC free article] [PubMed]
- Danno S, Itoh K, Matsuda T, Fujita J. Decreased expression of mouse Rbm3, a cold-shock protein, in Sertoli cells of cryptorchid testis. Am J Pathol. 2000;156:1685–1692. doi: 10.1016/S0002-9440(10)65039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, et al. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. American journal of physiology. Regulatory, Integrative and Comparative Physiology. 2008;295:R1263–1273. doi: 10.1152/ajpregu.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Vanderklish PW, Dupont-Versteegden EE. Enhanced survival of skeletal muscle myoblasts in response to overexpression of cold shock protein RBM3. Am J Physiol Cell Physiol. 2011;301:C392–402. doi: 10.1152/ajpcell.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisi V, Miseta A, Nagy T. The role of stress-induced O-GlcNAc protein modification in the regulation of membrane transport. Oxid Med Cell Longev. 2017;2017:1308692. doi: 10.1155/2017/1308692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S (2014) Synthetic lethality by co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling. Mitochondrion 19 Pt A:85–87 [DOI] [PubMed]
- Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol. 2017;34:49–55. doi: 10.1016/j.coph.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbraikh D, et al. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020;21:e48791. doi: 10.15252/embr.201948791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, et al. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun. 2017;8:1491. doi: 10.1038/s41467-017-01654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, et al. Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. American journal of physiology. Endocrinol Metab. 2015;308:E380–392. doi: 10.1152/ajpendo.00277.2014. [DOI] [PubMed] [Google Scholar]
- Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, et al. OGT upregulates myogenic IL-6 by mediating O-GlcNAcylation of p65 in mouse skeletal muscle under cold exposure. J Cell Physiol. 2022;237:1341–1352. doi: 10.1002/jcp.30612. [DOI] [PubMed] [Google Scholar]
- Huy H, et al. TXNIP regulates AKT-mediated cellular senescence by direct interaction under glucose-mediated metabolic stress. Aging Cell. 2018;17:e12836. doi: 10.1111/acel.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TC, et al. Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience. 2015;305:268–278. doi: 10.1016/j.neuroscience.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jögi A, et al. Nuclear expression of the RNA-binding protein RBM3 is associated with an improved clinical outcome in breast cancer. Modern Pathol : Official J United States and Canadian Acad Pathol Inc. 2009;22:1564–1574. doi: 10.1038/modpathol.2009.124. [DOI] [PubMed] [Google Scholar]
- Knott G. Neurodegeneration: cold shock protects the brain. Nature. 2015;518:177–178. doi: 10.1038/nature14195. [DOI] [PubMed] [Google Scholar]
- Kong F et al (2020) Cold exposure-induced up-regulation of Hsp70 positively regulates PEDV mRNA synthesis and protein expression in vitro. Pathogens (Basel, Switzerland) 9 9(4):246 [DOI] [PMC free article] [PubMed]
- Labbé SM, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB Journal : Off Publ Fed Am So Exp Biol. 2015;29:2046–2058. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- Lambert M, Bastide B, Cieniewski-Bernard C. Involvement of O-GlcNAcylation in the skeletal muscle physiology and physiopathology: focus on muscle metabolism. Front Endocrinol (lausanne) 2018;9:578. doi: 10.3389/fendo.2018.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebsack TW, et al. Microarray analysis of spaceflown murine thymus tissue reveals changes in gene expression regulating stress and glucocorticoid receptors. J Cell Biochem. 2010;110:372–381. doi: 10.1002/jcb.22547. [DOI] [PubMed] [Google Scholar]
- Lee JY, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- Lee A, et al. Combined antibody/lectin enrichment identifies extensive changes in the O-GlcNAc sub-proteome upon oxidative stress. J Proteome Res. 2016;15:4318–4336. doi: 10.1021/acs.jproteome.6b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, et al. Thimerosal-induced apoptosis in mouse C2C12 myoblast cells occurs through suppression of the PI3K/Akt/survivin pathway. PLoS ONE. 2012;7:e49064. doi: 10.1371/journal.pone.0049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, et al. Gas6 attenuates lipopolysaccharide-induced TNF-α expression and apoptosis in H9C2 cells through NF-κB and MAPK inhibition via the Axl/PI3K/Akt pathway. Int J Mol Med. 2019;44:982–994. doi: 10.3892/ijmm.2019.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. The protein phosphatase 1 complex is a direct target of AKT that links insulin signaling to hepatic glycogen deposition. Cell Rep. 2019;28:3406–3422.e3407. doi: 10.1016/j.celrep.2019.08.066. [DOI] [PubMed] [Google Scholar]
- Lian S, et al. Prenatal cold stress: effect on maternal hippocampus and offspring behavior in rats. Behav Brain Res. 2018;346:1–10. doi: 10.1016/j.bbr.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. RPS15A promotes gastric cancer progression via activation of the Akt/IKK-β/NF-κB signalling pathway. J Cell Mol Med. 2019;23:2207–2218. doi: 10.1111/jcmm.14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. O-GlcNAc / Akt pathway regulates glucose metabolism and reduces apoptosis in liver of piglets with acute cold stress. Cryobiology. 2021;100:125–132. doi: 10.1016/j.cryobiol.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. O-GlcNAcylation: the “stress and nutrition receptor” in cell stress response. Cell Stress Chaperones. 2021;26:297–309. doi: 10.1007/s12192-020-01177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Cold-induced RNA-binding protein promotes glucose metabolism and reduces apoptosis by increasing AKT Phosphorylation in mouse skeletal muscle under acute cold exposure. Front Mol Biosci. 2021;29(8):685993. doi: 10.3389/fmolb.2021.685993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, et al. RNA binding motif protein 3 (RBM3) drives radioresistance in nasopharyngeal carcinoma by reducing apoptosis via the PI3K/AKT/Bcl-2 signaling pathway. Am J Transl Res. 2018;10:4130–4140. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fleites C, et al. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat Struct Mol Biol. 2008;15:764–765. doi: 10.1038/nsmb.1443. [DOI] [PubMed] [Google Scholar]
- Matsuda A, et al. Generation of mice deficient in RNA-binding motif protein 3 (RBM3) and characterization of its role in innate immune responses and cell growth. Biochem Biophys Res Commun. 2011;411:7–13. doi: 10.1016/j.bbrc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Messmer MN, Kokolus KM, Eng JW, Abrams SI, Repasky EA. Mild cold-stress depresses immune responses: implications for cancer models involving laboratory mice. BioEssays : News Rev Mol Cellular Dev Biol. 2014;36:884–891. doi: 10.1002/bies.201400066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, et al. Evidence that the sympathetic nervous system elicits rapid, coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity during cold exposure. Diabetes. 2017;66:823–834. doi: 10.2337/db16-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellasdemunt L, et al. Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids. J Biol Chem. 2013;288:10640–10651. doi: 10.1074/jbc.M113.455998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, et al. Pivotal role of O-GlcNAc modification in cold-induced thermogenesis by brown adipose tissue through mitochondrial biogenesis. Diabetes. 2017;66:2351–2362. doi: 10.2337/db16-1427. [DOI] [PubMed] [Google Scholar]
- Pant M, Bal NC, Periasamy M. Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab. 2016;27:881–892. doi: 10.1016/j.tem.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MP, Peterson KR, Slawson C (2021) O-GlcNAcylation and O-GlcNAc cycling regulate gene transcription: emerging roles in cancer. Cancers 13(7):1666 [DOI] [PMC free article] [PubMed]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Peretti D, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015;518:236–239. doi: 10.1038/nature14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti D et al (2021) TrkB signaling regulates the cold-shock protein RBM3-mediated neuroprotection. Life Sci Alliance 4(4):e202000884 [DOI] [PMC free article] [PubMed]
- Rathjen NA, Shahbodaghi SD, Brown JA. Hypothermia and cold weather injuries. Am Fam Physician. 2019;100:680–686. [PubMed] [Google Scholar]
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Rodríguez-García A, et al. TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017;284:3437–3454. doi: 10.1111/febs.14201. [DOI] [PubMed] [Google Scholar]
- Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med. 2012;14:e1. doi: 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- Sepa-Kishi DM, Katsnelson G, Bikopoulos G, Iqbal A, Ceddia RB (2018) Cold acclimation reduces hepatic protein kinase B and AMP-activated protein kinase phosphorylation and increases gluconeogenesis in rats. Physiol Rep 6(5):e13592 [DOI] [PMC free article] [PubMed]
- Shi H, et al. Skeletal muscle O-GlcNAc transferase is important for muscle energy homeostasis and whole-body insulin sensitivity. Molecular Metabolism. 2018;11:160–177. doi: 10.1016/j.molmet.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, et al. Regulating glycolysis, the TLR4 signal pathway and expression of RBM3 in mouse liver in response to acute cold exposure. Stress (amsterdam, Netherlands) 2019;22:366–376. doi: 10.1080/10253890.2019.1568987. [DOI] [PubMed] [Google Scholar]
- Short B. Cold temperatures put a freeze on myosin activation. J Gen Physiol. 2019;151:1247. doi: 10.1085/jgp.201912509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo Y, et al. Identification of a novel enhancer that binds Sp1 and contributes to induction of cold-inducible RNA-binding protein (cirp) expression in mammalian cells. BMC Biotechnol. 2012;12:72. doi: 10.1186/1472-6750-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureban SM, et al. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27:4544–4556. doi: 10.1038/onc.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, et al. Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices. Brain Res. 2013;1504:74–84. doi: 10.1016/j.brainres.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Ushio A, Eto K. RBM3 expression is upregulated by NF-κB p65 activity, protecting cells from apoptosis, during mild hypothermia. J Cell Biochem. 2018;119:5734–5749. doi: 10.1002/jcb.26757. [DOI] [PubMed] [Google Scholar]
- Van Pelt DW, Confides AL, Judge AR, Vanderklish PW, Dupont-Versteegden EE. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J Muscle Res Cell Motil. 2018;39:35–40. doi: 10.1007/s10974-018-9496-x. [DOI] [PubMed] [Google Scholar]
- Venugopal A, et al. RNA binding protein RBM3 increases β-catenin signaling to increase stem cell characteristics in colorectal cancer cells. Mol Carcinog. 2016;55:1503–1516. doi: 10.1002/mc.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wahl R. Responses of the insulin signaling pathways in the brown adipose tissue of rats following cold exposure. PLoS ONE. 2014;9:e99772. doi: 10.1371/journal.pone.0099772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia. 2016;59:1287–1296. doi: 10.1007/s00125-016-3919-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 2020;21:e50085. doi: 10.15252/embr.202050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann S, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117:1785–1794. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- Wellmann S, et al. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr Res. 2010;67:35–41. doi: 10.1203/PDR.0b013e3181c13326. [DOI] [PubMed] [Google Scholar]
- Wong JJ, et al. RBM3 regulates temperature sensitive miR-142-5p and miR-143 (thermomiRs), which target immune genes and control fever. Nucleic Acids Res. 2016;44:2888–2897. doi: 10.1093/nar/gkw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. Cold stress regulates lipid metabolism via AMPK signalling in Cherax quadricarinatus. J Therm Biol. 2020;92:102693. doi: 10.1016/j.jtherbio.2020.102693. [DOI] [PubMed] [Google Scholar]
- Xia W, Su L, Jiao J. Cold-induced protein RBM3 orchestrates neurogenesis via modulating Yap mRNA stability in cold stress. J Cell Biol. 2018;217:3464–3479. doi: 10.1083/jcb.201801143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, et al. O-GlcNAc modification of NFκB p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS ONE. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, et al. RNA-binding protein RBM3 prevents NO-induced apoptosis in human neuroblastoma cells by modulating p38 signaling and miR-143. Sci Rep. 2017;7:41738. doi: 10.1038/srep41738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699–707. doi: 10.1016/j.biopha.2018.04.072. [DOI] [PubMed] [Google Scholar]
- Yao R et al (2018) Effects of acute cold stress on liver O-GlcNAcylation and glycometabolism in mice. Int J Mol Sci 19(9):2815 [DOI] [PMC free article] [PubMed]
- Yuan X, et al. Expression regulation of cold-inducible protein RBM3 by FAK/Src signaling for neuroprotection against rotenone under mild hypothermia. Biochem Biophys Res Commun. 2021;534:240–247. doi: 10.1016/j.bbrc.2020.11.105. [DOI] [PubMed] [Google Scholar]
- Zhang CS, et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548:112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chai W, Xiang Z, Zhou X, Zhang P. MZF1 alleviates oxidative stress and apoptosis induced by rotenone in SH-SY5Y cells by promoting RBM3 transcription. J Toxicol Sci. 2021;46:477–486. doi: 10.2131/jts.46.477. [DOI] [PubMed] [Google Scholar]
- Zhang D et al (2015) OGT-mediated O-GlcNAcylation promotes NF-κB activation and inflammation in acute pancreatitis. Inflammation research 64:943–952 [DOI] [PubMed]
- Zhao FQ, et al. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19:635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cellular and Molecular Life Sciences : CMLS. 2016;73:3839–3859. doi: 10.1007/s00018-016-2253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zelmer A, Kapfhammer JP, Wellmann S. Cold-inducible RBM3 inhibits PERK phosphorylation through cooperation with NF90 to protect cells from endoplasmic reticulum stress. FASEB Journal : Official Publ Fed Am Soc Exp Biol. 2016;30:624–634. doi: 10.1096/fj.15-274639. [DOI] [PubMed] [Google Scholar]
- Zhuang RJ, et al. Cold-inducible protein RBM3 protects UV irradiation-induced apoptosis in neuroblastoma cells by affecting p38 and JNK pathways and Bcl2 family proteins. J Molec Neurosci : MN. 2017;63:142–151. doi: 10.1007/s12031-017-0964-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding authors on reasonable request.