Abstract
Purpose
In this systematic review and meta-analysis, we investigated the effect and side effects of Citrullus colocynthis on glycemic factors and lipid profile in diabetic patients.
Methods
We systematically searched English and Persian databases from inception till August 2021 using Medical Subject Headings (MeSH). Two authors independently extracted data and assessed the quality of studies. The standardized mean differences were pooled using fixed-effect models, and statistical heterogeneity was assessed using the I squared (I²) index.
Results
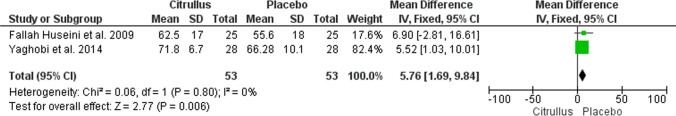
Of the 321 articles searched in the databases, 136 related articles were screened, 14 relevant full-text articles were assessed for eligibility; finally, four articles were included in the study, three articles were entered into the meta-analysis. The results of the meta-analysis indicated that Citrullus colocynthis does not have a significant effect on fasting blood sugar (FBS), hemoglobin A1c (HBA1c), low-density lipoprotein (LDL), total cholesterol, and triglyceride indices but increases high-density lipoprotein (HDL) (Mean Difference: 5.76; 95% CI: 1.69 to 9.84; P = 0.006; I2 = 0%).
Conclusions
The meta-analysis results showed that Citrullus colocynthis has no significant effect on glycemic and metabolic indices of diabetes - except HDL. Due to the relatively low quality and the small number of included trials, conducting further large scale well-designed randomized clinical trials to determine the effect of Citrullus colocynthis on glycemic and metabolic indices seems essential.
Supplementary information
The online version contains supplementary material available at 10.1007/s40200-022-01045-9.
Keywords: Citrullus colocynthis, Diabetes mellitus, Hyperglycemia, Systematic review, Clinical trial
Introduction
Diabetes mellitus (DM) is a chronic disease caused by lack of insulin or insulin dysfunction resulting in hyperglycemia [1]. It is one of the most common diseases and is becoming pandemic in many parts of the world. According to studies, around 422 million people worldwide are suffering from the disease, and it is predicted that by the year 2035, approximately 592 million people will be affected by the disease, and about 300 million people will die of diabetes [2]. The disease affects about 4.4 million people in Iran between the ages of 25 and 64, and about 16.8% of Iran’s adult population has a fasting glucose disorder [3].
The disease is divided into diabetes mellitus type 1 and type 2 [4]. The mechanisms in type 2 Diabetes mellitus (T2DM) involve a series of environmental and genetic risk factors that reduce peripheral insulin sensitivity and beta-cell function [5]. The most important risk factors for developing type 2 diabetes include having an unhealthy diet, a sedentary lifestyle, a genetic predisposition to the disease as well as living in an environment that promotes diabetes development [6].
Besides modifying the diet and increasing physical activity, there are several types of oral medications to treat T2DM, which are used alone or in combination. These include metformin, thiazolidinediones, dopamine agonists, bile acid sequestrants, meglitinides, alpha-glucosidase inhibitors, dipeptidyl peptidase IV (DPP-4) inhibitors, sodium-glucose transport protein 2 (SGLT2) inhibitors, and oral glucagon-like peptide 1 (GLP-1) receptor agonists [7].
Because of the high prevalence of diabetes and its associated complications [1, 8, 9] in recent years, there has been a focus on herbal medicine, which can offer the benefit of having fewer side effects compared to conventional therapeutic interventions listed above [3].
Citrullus colocynthis is a plant used by traditional medicine practitioners to treat diabetes in Iran [10]. The fruit of this plant has different medical uses in traditional Persian medicine, i.e., anti-inflammatory, purgative, antidiabetic, analgesic, hair growth-promoting, and antiepileptic. However, some of the side effects of this plant have been documented in modern medicine, such as nausea, vomiting, diarrhea, colic, hematochezia, and nephrosis [11].
Since diabetes is a highly prevalent disease that negatively affects the quality of life [1, 9, 12], it is necessary to assess the effect of Citrullus colocynthis due to its better affordability. Several clinical trials have demonstrated the efficacy of Citrullus colocynthis in reducing hemoglobin A1c and fasting blood glucose [8, 10, 13–17]. However, according to our investigation, studies have not yet reached a comprehensive conclusion about the effect of this plant, and there is no specific systematic review that assessed all these criteria. Therefore, this systematic review was conducted to determine the effect of Citrullus colocynthis fruit on the glycemic indices and lipid profile in T2DM.
Methods
Search strategy
In this systematic review, we investigated randomized controlled clinical trials on the effect of Citrullus colocynthis on glycemic and metabolic parameters of patients with T2DM. The search was conducted in February 2021 without publication time restriction for published or ongoing articles. We searched all Persian and English articles in the following databases: Medline (via PubMed), Scopus, Embase (via Ovid), Cochrane Library, Web of Sciences, ProQuest, Google Scholar, ClinicalTrials.gov, SID, Magiran, Irandoc, and Iranmedex. The search strategy was presented in the PRISMA chart. We searched gray literature and references of related articles as well. The search strategy of the articles in this study was based on the MeSH Glossary. The following keywords were used alone or cross-linked, depending on which database would be used, including “diabetes,“ “Citrullus colocynthis,“ “Colocynth,“ “blood glucose,“ “glucose,“ “hyperglycemia,“ “insulin,“ “insulin resistance,“ and “clinical trials.“ The acceptability of the articles was evaluated separately by two authors (AJ, AR). If there was a conflict between the authors, the study was first discussed, and in case of disagreement, they consulted with the third author (NGA).
Study selection
PICOS
The PICOS pattern, which includes (Participants, intervention, comparison, outcomes, and study design) was followed:
Participants
In the present study, the participants included All 18 to 75-year-old men and women with T2DM (patients diagnosed with T2DM 1 to 8 years ago) whose fasting blood sugar (FBS) was in the range of 125 to 200 mg / dL or those with a fasting blood cholesterol above 200 mg / Dl and an FBS between 160 and 200 mg / dL, none of whom were users of herbal medications. The following groups were excluded from the study: All type 1 diabetic patients, diabetic patients with liver, kidney, neurological, cardiovascular, or gastrointestinal diseases, patients with a history of gastrointestinal surgery, type 2 diabetic patients who take insulin, and pregnant or breastfeeding women.
Intervention
The intervention group took Citrullus colocynthis as tablets, capsules, or oral drops with different doses for 30 to 60 days. The studies that examined the non-oral consumption of Citrullus colocynthis were excluded from the present study. The comparison group comprised a placebo group. It should be noted that both intervention and comparison groups used their previous drugs (metformin 500 and glibenclamide 5). Patients receiving insulin therapy were excluded from the study.
Outcomes
Primary outcomes included fasting serum glucose and hemoglobin A1c, triglycerides, LDL, and HDL. Furthermore, the secondary consequences were the side effects of Citrullus colocynthis, measured by indicators of hepatic enzymes, serum creatinine, and serum urea.
Study design
All types of clinical trials, including crossover, before and after studies, and randomized controlled clinical trials about the effect of Citrullus colocynthis on glycemic and metabolic parameters of patients with T2DM were evaluated in this study.
Data extraction and quality assessment of included studies
The two authors (AJ, AR) separately reviewed the acceptability and quality of the articles. If there were a contradiction between the two authors, the disagreement would be resolved by consultation with the third person (NGA). If we confronted the ambiguity of the collected data, we would have attempted to contact the article’s authors to get further information. To evaluate the quality of articles for randomized clinical trials, we used the “collaborations tool for assessing the risk of bias in randomized trials” [18]. This is a standard tool used to evaluate clinical trials’ methodological quality to detect the following biases: selection bias, performance bias, detection bias, attrition bias, and reporting bias. The data extraction form was designed based on the Cochrane handbook for systematic reviews of interventions [19]. We also extracted study-related data including the year of publication, country of study, the first author’s name, methodology, type and method of consumption of Citrullus colocynthis, control group, duration of treatment, the follow-up period, characteristics of participants, number of randomized participants, primary and secondary outcomes, and reported side effects.
Statistical analysis
Statistical analysis complied with guidelines determined in the Cochrane handbook for the systematic review of interventions. If the outcome measurement in the included studies was sufficiently homogeneous, we conducted a quantitative synthesis. Statistical homogeneity was assessed using I squared (I²) statistics.
If trials were clinically comparable in terms of participants, intervention, comparator, outcomes, and measurement period, we combined each trial’s result in a meta-analysis using review manager software (RevMan version 5.3) [20]. We extracted the mean and standard deviation (SD) of pre-specified outcomes from each study.
Continuous outcome data, measured with the same instrument in each included study, reported a mean difference (MD) and a 95% confidence interval using the fixed-effect model.
Results
Study selection
A total of 321 studies were identified from the initial search. After removing duplicated records (n = 136), 185 articles remained. Through screening titles and abstracts of remaining records, 171 studies were excluded for the following reasons: not relevant to Citrullus colocynthis, observational studies, animal experiments, and pharmacological studies. Then, the full texts of the remaining articles (n = 14) were assessed for eligibility. Ten full-text articles were excluded due to the following reason: they did not consider the desired outcome in the intended population. Some studies were excluded from the review for the following reasons: Rahbar et al.‘s [21] study for lack of inclusion criteria and non-diabetic samples, the study of Hassan Mukhtiar et al. [15] for the unreasonable similarity of the data with the Li et al. [16] and the duplication of the reported data between the two studies, the study of Ahangarpour et al. [22] due to investigating dermal absorption and not the oral form of the plant and the absence of placebo group, and finally two Persian studies of Fallah Huseini et al. [13, 14] in 2006 because of the similarity of the data to his article [10] in 2009. In addition to these studies, an unpublished protocol on the IRCT site also studied the effect of dermal use of Citrullus colocynthis gel on reducing glycemic index in diabetic patients. We contacted the author to collect the study’s data, but we did not receive any response [23]. Finally, four articles were included in the systematic review, and three articles were selected for the meta-analysis [8, 10, 17]. Two authors separately reviewed the title and abstract of all studies. The flowchart of the study selection process is shown in Fig. 1.
Fig. 1.
Study flow diagram
Methodological quality
The systematic review included randomized controlled trials (RCTs) [8, 10, 16, 17]. In Li et al.‘s [16] study, allocation concealment was reported high risk because there was nothing documented about drug allocation or the placebo; Additionally, reporting bias, attrition bias, detection bias, performance bias, and selection bias were reported as unclear in that study. Yaghoobi’s study [17] was conducted using the method of sequencing. Two studies [10, 17] reported randomization (using a similar matte envelope method). All three trials were double-blind studies [8, 10, 17]. All studies reported participant withdrawal, but only in the protocol of two studies [8, 17], the reporting bias was mentioned. By comparing the articles and their protocols, we found that some consequences were not reported, so we contacted the authors in charge, but we did not receive any response. We did not find another study’s protocol [10], and that study’s risk was reported as unknown. The risk of bias of all studies is shown in Tables 1 and 2; Figs. 2 and 3.
Table 1.
Characteristics of included studies
| First author | Type of clinical trial | Sample size | Age of participants (years) | Intervention (dosage + months of treatment) | Comparison (dosage + months of treatment) | Duration of follow | Outcomes | Results | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Fallah Huseini et al. (2009) | RCTa |
50 Citrullus colocynthis group: 25 Placebo group: 25 |
40–65: type 2 diabetes | Citrullus colocynthis capsule 100 mg three times a day for 2 months | Placebo capsule two times a day for 2 months | 0 |
Glycosylated hemoglobin (HbA1ck), FBSC, Chold, LDLf, HDLg, TGe (Main), aspartate transaminase, alanine transaminase, alkaline phosphatase, urea, and creatinine (Secondary) |
The results showed a significant decrease in HbA1c and fasting blood glucose levels in Citrullus colocynthis treated patients. Other serological parameters levels in both groups did not change significantly. | No notable gastrointestinal side effect was observed in either group. |
|
Yaghobi et al. (2014) |
RCTa-double blinding |
60 Citrullus colocynthis group: 28 Placebo group: 28 |
30–60: type 2 diabetes with hyperlipidemia | Citrullus colocynthis capsule 100 mg three times a day for 1 month | Placebo capsule three times a day for 1 month | Not mentioned |
BSb, Chold, TGe, LDLf, HDLg (Main) ASTh, Crj, ALTi (Secondary) |
The results suggest that processed Citrullus colocynthis fruit extract may be a safe anti-hyperglycemic and anti-hypercholesterolemic agent in hyperlipidemic type II diabetic patients. | No adverse effect was observed with Citrullus colocynthis |
| Barghamdi et al. (2016) | RCTa |
70 Citrullus colocynthis group: 35 Placebo group: 35 |
40–65 : type 2 diabetes | Citrullus colocynthis capsule 125 mg once per day just before lunch meal for 2 months | Placebo capsule once per day for 2 months | 0 | FBSc, HbA1ck | This study showed that Citrullus colocynthis fruit has a considerable effect on reduction in the mean serum level of HbA1c and FBS in patients with the type II diabetes | No adverse effect was observed with Citrullus colocynthis |
| Youshan Li et al. 2015 | RCTa |
32 Citrullus colocynthis group: 8 Placebo group: 8 Gymnema sylvestre: 8 Artemisia absinthium: 8 |
30–60 : type 2 diabetes | Citrullus colocynthis capsule 500 mg two times a day for 1 month | Placebo capsule two times a day for 1 month | 10 days | FBSc, Chold, TGe, LDLf, HDLg |
Citrullus colocynth reduced glucose, cholesterol and TG and HDL-cholesterol levels by 35, 6, 6, and 5%, respectively. Good antidiabetic features, however these herbal products had no significant effect on lipid profiles of the diabetic human. |
The side effect was not mentioned in this study |
a RCT: Randomized Controlled Trial, b BS: Blood Sugar, c FBS: Fasting Blood Sugar, d Chol: cholesterol, e TG: Triglyceride, f LDL: low-density lipoprotein, g HDL: High-density lipoprotein, h AST: Aspartate Aminotransferase, i ALT: Alanine aminotransferase, j Cr: Creatinine, k HbA1c: glycosylated hemoglobin
Table 2.
Risk of bias assessment in included studies
| Bias | Authors’ judgment | Support for judgment |
|---|---|---|
| Fallah Huseini et al. | ||
| Random sequence generation (selection bias). | Unclear | No randomization method described |
| Allocation concealment (selection bias) | Low risk | The medications were placed in two boxes, and no difference could be detected. |
| Blinding of participants and personnel (performance bias) | Low risk | Double-blind |
| Blinding of outcome assessment (detection bias). | low risk | Double-blind |
| Incomplete outcome data (attrition bias). | Low risk | No loss |
| Selective reporting (reporting bias). | Unclear | Not found |
| Barghamdi et al. | ||
| Random sequence generation (selection bias). | Unclear | No randomization method was described. |
| Allocation concealment (selection bias) | Unclear | The method of concealment was not described in sufficient detail to allow a definite judgment. |
| Blinding of participants and personnel (performance bias) | Low risk | Double-blind |
| Blinding of outcome assessment (detection bias). | Low risk | Double-blind |
| Incomplete outcome data (attrition bias). | Low risk | No loss |
| Selective reporting (reporting bias). | High risk | LDL, HDL, cholesterol and triglyceride not reported |
| Yaghobi et al. | ||
| Random sequence generation (selection bias). | Low risk |
Randomized selecting the A and B codes by the patient and recording in the file |
| Allocation concealment (selection bias) | Low risk | The medications were placed in two boxes, and no difference could be detected. |
| Blinding of participants and personnel (performance bias) | Low risk | Double-blind |
| Blinding of outcome assessment (detection bias). | Low risk | Double-blind |
| Incomplete outcome data (attrition bias). | Low risk | Minimal loss |
| Selective reporting (reporting bias). | High risk | HbA1c not reported |
| Li et al. | ||
| Random sequence generation (selection bias). | Unclear risk | No randomization method described |
| Allocation concealment (selection bias) | Unclear risk | The concealment method has not been described in sufficient detail to allow us to reach an accurate conclusion. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias). | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias). | Unclear risk | Not mentioned |
| Selective reporting (reporting bias). | Unclear risk | The protocol’s database was not found. |
Fig. 2.

Risk of bias summary: review authors ҆ judgment about each risk of bias item for each included study
Fig. 3.
The effect of Citrullus colocynthis compared to placebo on FBS
Description of studies
The clinical trials that examined the effect of Citrullus colocynthis on glycemic and metabolic indices are as follows:
In a study conducted by Fallah Huseini et al. [10] for two months on two groups of 25 patients with T2DM, the first group was given 100 mg capsules of Citrullus colocynthis fruit; the second group was given 100 mg capsules of a placebo, and each group took them three times a day. The study population comprised type 2 diabetic patients between 40 and 65 identified by the American Diabetes Association (ADA) criteria who were on a diabetic diet for at least two months and received no herbal medicine. The results showed Citrullus colocynthis had a significant effect on (p < 0.003) HbA1c and (p < 0.015) FBS but had no significant impact on other serological indices. No gastrointestinal or renal complications were seen.
Yaghoobi et al. [17] conducted a study in 2015 on two groups of 28 patients with T2DM and hyperlipidemia who ranged from 30 to 60 years in age. They were treated with two tablets of metformin (500 mg) or two tablets of glibenclamide (5 mg). They used Citrullus colocynthis 100 mg capsule three times a day. The results showed that the levels of fasting serum glucose (p = 0.019), cholesterol (p = 0.035), and LDL (p = 0.031) significantly decreased compared to the placebo group.
In a study conducted by Li et al. [16] in 2015, 32 patients with type 2 diabetes between 30 and 60 years, were divided into four groups. In this study, the first and third groups received one-gram capsules of Gymnema Sylvestre and Artemisia absinthium L, and the second group were prescribed a one-gram capsule of Citrullus colocynthis fruit daily for 30 days and were followed up for 10 days after the intervention. In patients who used Citrullus colocynthis FBS, cholesterol, TGL, and HDL levels improved in the intervention period compared to the follow-up period, in which the FBS reduction was significant (p < 0.05).
In 2016, Barghamdi et al. [8] studied 70 patients with T2DM aged 40 to 65 years, who had hemoglobin A1c above 7% in 3 consecutive tests. They divided this group into two subgroups and studied for two months. The intervention group was given 125 mg capsules of Citrullus colocynthis. At the end of 2 months, fasting serum glucose (P = 0.04) and hemoglobin A1C (P = 0.01) in the intervention group were significantly lower than in the placebo group. This study also showed a negative relationship between FBS levels (P = 0.03) and HbA1c (P = 0.008) with patients’ body mass index. No side effects were reported during this study. The general characteristics and methodological characteristics of each study are shown in Table 1.
Meta-analysis results
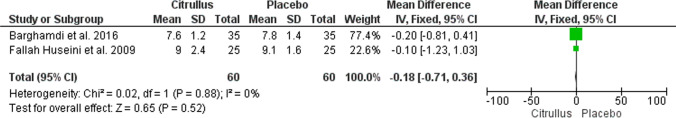
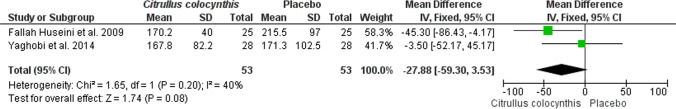
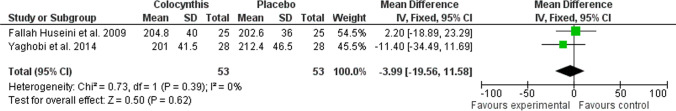
In this systematic review, three articles studied the effect of Citrullus colocynthis on glycemic and metabolic parameters of patients with T2DM. Therefore, all three were selected for meta-analysis [8, 10, 17]. The meta-analysis results showed that the consumption of Citrullus colocynthis in the intervention group in comparison to the control group did not significantly affect the level of the glycemic and metabolic indices, including FBS (n = 3 studies; MD: -4.31; 95% CI: -17.89 to -9.27; P = 0.53; I2 = 0%) (Fig. 3), HbA1c (n = 2 studies; MD: -0.18; 95% CI: -0.71 to 0.36; P = 0.52; I2 = 0%) (Fig. 4), LDL (n = 2 studies; MD: -6.66; 95% CI: -17.52 to 4.19; P = 0.23; I2 = 1%) (Fig. 5), total cholesterol (n = 2 studies; MD: -3.99; 95% CI: -19.56 to 11.58; P = 0.62; I2 = 0%) (Fig. 6), triglycerides (n = 2 studies; MD: -27.88; 95% CI: -59.30 to 3.53; P = 0.08; I2 = 40%) (Fig. 7) except HDL (n = 2 studies; MD: 5.76; 95% CI: 1.69 to 9.84; P = 0.006; I2 = 0%) (Fig. 8) which showed an improvement.
Fig. 4.
The effect of Citrullus colocynthis compared to placebo on HbA1c
Fig. 5.
The effect of Citrullus colocynthis compared to placebo on LDL
Fig. 7.
The effect of Citrullus colocynthis compared to placebo on triglycerides
Fig. 8.
The effect of Citrullus colocynthis compared to placebo on HDL
Fig. 6.
The effect of Citrullus colocynthis compared to placebo on total cholesterol
Side effects
Fallah Huseini and Yaghoobi [10, 17] studied liver and kidney complications, including AST, ALT, and serum creatinine. Fallah Huseini [10] also studied alkaline phosphatase and serum urea. The urea in the intervention group increased compared to the comparison group, and the alkaline phosphatase decreased in the intervention group compared to the placebo group. No significant changes have been shown in a meta-analysis of ALT, AST, and creatinine. No serious side effects of this plant were reported in the studied articles.
Discussion
Nowadays, 422 million people worldwide are living with diabetes mellitus, and this number is increasing [2]. Due to the costs involved in managing diabetes and its complications, many physicians and researchers are seeking complementary and traditional medicine. In traditional medicine, Citrullus colocynthis is one of the plants that has been mentioned a lot in Persian medicine papers [24–27].
In this systematic review, we aimed to survey the efficacy and safety of Citrullus colocynthis on serum glycemic indices and lipid profile of T2DM patients. The present study indicated that the consumption of Citrullus colocynthis compared to the placebo did not significantly affect the level of the glycemic and metabolic indices except for the level of HDL, which was increased in the intervention group. However, all three articles entered into this meta-analysis separately showed that Citrullus colocynthis consumption could reduce FBS [8, 10, 17]. HbA1c was significantly reduced in two studies [8, 10]. LDL and total cholesterol were reduced in one study [17].
So far, many studies on the effects of Citrullus colocynthis as antidiabetic, laxative, anti-inflammatory, hyperlipidemic, antibiotic, anti-helminth, epilepsy reliever, knee, sciatica, and ear pain reliever have been conducted. Citrullus colocynthis also has been used for gout, colic treatment, and hair loss prevention [11, 21, 24–32]. The main chemical composition of the pulp contains pectin, colocynthin, colocynthein, colocynthetin, citrullol, elaterin, elatericin B, and gum. The seeds of this plant contain stabilized oils and albinoids [11].
The results of animal and laboratory studies on the effectiveness of Citrullus colocynthis on glycemic factors are not compatible with the present study results. The compounds of this plant, with rich sources of tinosporine, cordifolide, tinosporide, cordifole, affect cholesterol synthesis and glycolysis. On the other hand, the effect of these compounds on insulin secretion and glycogen synthesis has also been observed [33]. Citrullus colocynthis plant decreases the amount of HbA1c in the long period by prolonging the synthesis time of HbA1c in the laboratory, which plays a useful role in lowering glycosylated proteins [34]. In colocynth, there are compounds such as phytocompounds, polyphenols, and flavonoids that play the function of removing free radicals and thus affect diabetes [35].
Some other human studies also did not match the present study results. A clinical trial that examined the non-oral consumption of Citrullus colocynthis in diabetic patients [22] showed a significant decrease in blood glucose and serum urea indices compared to pre-intervention levels. This plant’s dermal uptake was reported to stimulate insulin secretion and improve pancreatic beta cells’ activity, but no significant changes were observed in serum creatinine levels, lipid profiles, liver enzymes, urinary microalbumin, and insulin sensitivity indices. In the article of Li et al. [16], which was excluded from the meta-analysis due to non-reporting of placebo group data, the results showed Citrullus colocynthis reduced glucose, cholesterol, TGL, and HDL in T2DM patients.
Several reasons caused differences between the present study results and animal, human, and traditional medicine recommendations. Most meta-analyses were performed based on the results of two or three studies which their small sample size and large standard deviation had affected the final results of these meta-analyses. On the other hand, some articles’ low quality exposes the results of these studies to various biases. Also, no follow-up was performed in the three studies. Therefore, it is challenging to reach a definitive conclusion regarding the effect of Citrullus colocynthis on diabetes mellitus.
Few studies evaluate the side effects of this plant. Like most herbal medicines, the concern about possible hepatic and renal complications resulting from Citrullus colocynthis consumption was substantial; However, our meta-analysis did not demonstrate such complications. Other side effects of this plant, such as acute rectorrhagia [36] and acute interstitial nephritis [37], were not reported in studies included in this systematic review. In an animal study, a 12-week use of this plant has been shown to reduce pregnancy and zygote implantation in Sprague-Dawley rats [38]. In another animal study, diarrhea, leukopenia, anemia, AST, ALT and alkaline phosphatase changes, serum protein, serum albumin, urea, and bilirubin, as well as changes in liver fat and liver damage, were seen [39].
Strengths and limitations
In this systematic review, the effect of Citrullus colocynthis on glycemic and metabolic indices of diabetes in type 2 diabetic patients was reviewed for the first time. Citrullus colocynthis is one of Iran’s regional plants. We were able to access studies published in Persian, which would be of tremendous benefit given the regional nature of Citrullus colocynthis. However, we did not discover any non-English and Persian articles in other countries which grow this plant, such as Tunisia. Studies included in this meta-analysis were of limited value because they were conducted using small sample sizes and were arguably prone to many biases. These factors demonstrate that articles involved in this meta-analysis and systematic review were incapable of providing conclusive results. None of the articles involved a long-term follow-up of participants.
Conclusions
The present study results demonstrated that consumption of Citrullus colocynthis in the intervention group compared to the control group had no significant effect on FBS, HbA1c, LDL, triglycerides, total cholesterol but improved HDL levels. However, due to the relatively low quality of articles and the limited number of studies, it is necessary to conduct well-designed clinical trials to determine Citrullus colocynthis’ effect on glycemic and metabolic parameters of diabetes in type 2 diabetic patients. Side effects with the use of this plant were not seen in any of the studies, but due to the limited follow-up after the intervention and the small number of participants, more studies are needed to consider this plant as a safe medication.
Statement of authorship
Conceptualization: Ali Jafarizadeh, Seyed Amin Raeisi, Nafiseh Ghassab-Abdollahi, Reza Yarani, Mostafa Araj-Khodaei, Mojgan Mirghafourvand; Search strategy and data extraction: Ali Jafarizadeh, Seyed Amin Raeisi, Nafiseh Ghassab-Abdollahi; Formal analysis: Nafiseh Ghassab-Abdollahi, Mojgan Mirghafourvand; Supervision: Reza Yarani, Mostafa Araj-Khodaei, Mojgan Mirghafourvand.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 31 KB)
Declarations
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol. 2014;126:211–22. doi: 10.1016/B978-0-444-53480-4.00015-1. [DOI] [PubMed] [Google Scholar]
- 2.Kreider KE, Gabrielski AA, Hammonds FB. Hyperglycemia Syndromes. Nurs Clin North Am. 2018;53(3):303–17. doi: 10.1016/j.cnur.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31(1):96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 4.Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. 2019;13(1):364–72. doi: 10.1016/j.dsx.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Baker JR, O’Connor JP, Metcalf PA, Lawson MR, Johnson RN. Clinical usefulness of estimation of serum fructosamine concentration as a screening test for diabetes mellitus. Br Med J. 1983;287(6396):863–7. doi: 10.1136/bmj.287.6396.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arneth B, Arneth R, Shams M. Metabolomics of Type 1 and Type 2 Diabetes. Int J Mol Sci. 2019;20(10):2467. doi: 10.3390/ijms20102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feingold KR. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes. 2021 Aug 28. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–.
- 8.Barghamdi B, Ghorat F, Asadollahi K, Sayehmiri K, Peyghambari R, Abangah G. Therapeutic effects of Citrullus colocynthis fruit in patients with type II diabetes: A clinical trial study. J Pharm Bioallied Sci. 2016;8(2):130–4. doi: 10.4103/0975-7406.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi Y, Baradaran HR, Djalalinia S, Chinekesh A, Khamseh ME, Dastoorpoor M, et al. Complications of type 2 diabetes in Iranian population: An updated systematic review and meta-analysis. Diabetol Metab Syndr. 2019;13(3):2300–12. doi: 10.1016/j.dsx.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of Type II diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23(8):1186–9. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi R, Amin G, Ardekani MR. A review on Citrullus colocynthis Schrad.: from traditional Iranian medicine to modern phytotherapy. J Altern Complement Med. 2012;18(6):551–4. doi: 10.1089/acm.2011.0297. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21(3):617–38. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Fallah Huseini H, Heshmat R, Larijani B, Fakhrzadeh H, Jafariazar Z, Darvishzadeh F, et al. The clinical investigation of Citrullus colocynthis (L.) Schrad. fruit in treatment of type II diabetic patients a randomized, double-blind, placebo-controlled study. J Med Plants. 2006;1(17):31–5. doi: 10.1002/ptr.2754. [DOI] [PubMed] [Google Scholar]
- 14.Fallah Huseini H, Zaree A, Heshmat R, Larijani B, Fakhrzadeh H, Rezaii Sharifabadi R, et al. The effect of Citrullus colocynthis (L.) Schrad. fruit on oxidative stress parameters in type II diabetic patients. J Med Plants. 2006;1(17):55–60. [Google Scholar]
- 15.Hassan M, Niazi AT, Khan S, Gul F. Antidiabetic and antihyperlipidemic effects of artemisia absinthium l., citrullus colocynthis (l.) schrad. and gymnema sylvestre (retz.) r.br. ex sm. on type II diabetes hyperlipidemic patients. Indian J Tradit Knowl. 2018;17:233–9. [Google Scholar]
- 16.Li Y, Zheng M, Zhai X, Huang Y, Khalid A, Malik A, et al. Effect of-gymnema sylvestre, citrullus colocynthis and artemisia absinthium on blood glucose and lipid profile in diabetic human. Acta Pol Pharm. 2015;72(5):981–5. [PubMed] [Google Scholar]
- 17.Yaghoobi M, Miri-Moghaddam E, Navidian A, Nikbakht R, Mehrafarin A, Fallah Huseini H. Safety and Efficacy of processed Citrullus colocynthis L. Fruit in treatment of hyperlipidemic type II diabetic patients: a randomized, placebo-controlled clinical trial. J Med Plants. 2014;4(52):81–8. [Google Scholar]
- 18.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011;343.
- 20.Cochrane Collaboration . Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 21.Rahbar AR, Nabipour I. The hypolipidemic effect of Citrullus colocynthis on patients with hyperlipidemia. Pak J Biol Sci. 2010;13(24):1202–7. doi: 10.3923/pjbs.2010.1202.1207. [DOI] [PubMed] [Google Scholar]
- 22.Ahangarpour A, Belali R, Bineshfar F, Javadzadeh S, Yazdanpanah L. Evaluation of skin absorption of the Citrullus colocynthis in treatment of type II diabetic patients. J Diabetes Metab Disord. 2020;19(1):305–9. doi: 10.1007/s40200-020-00509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjari M. Evaluation of the efficacy of gel and aqueous extract of Citrullus colocynthis (L.) Schrad in patients with type 2 diabetes referring to Kerman diabetes clinic. 2018.
- 24.M. H. Alabnya an al-Haghayegh al-Advia [in Persian]. Tehran: Tehran University; 1992.
- 25.M. R. Alhavi fi-al-tib [in Arabic]. Beirut: Dar Al_Kotob Al-ilmiyah; 2000.
- 26.Tehran . Tohfeh al-Momenin [in Persian] Iran: Shahid Beheshti University of Medical Sciences; 2007. [Google Scholar]
- 27.Sina I. Al Qanun Fi al-Tibb Persian translated by Sharafkandi A. Tehran: Soroush Press; 2005.
- 28.Daradka H, Almasad MM, Qazan W, El-Banna NM, Samara OH. Hypolipidaemic effects of Citrullus colocynthis L. in rabbits. Pak J Biol Sci. 2007;10(16):2768–71. doi: 10.3923/pjbs.2007.2768.2771. [DOI] [PubMed] [Google Scholar]
- 29.Dhanotia R, Chauhan NS, Saraf DK, Dixit VK. Effect of Citrullus colocynthis Schrad fruits on testosterone-induced alopecia. Nat Prod Res. 2011;25(15):1432–43. doi: 10.1080/14786410802632820. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Kumar D, Manjusha, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58(2):215–20. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 31.Marzouk B, Marzouk Z, Décor R, Edziri H, Haloui E, Fenina N, et al. Antibacterial and anticandidal screening of Tunisian Citrullus colocynthis Schrad. from Medenine. J Ethnopharmacol. 2009;125(2):344–9. doi: 10.1016/j.jep.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 32.MH. A. Makhzan-al-Advia [in Persian]. Tehran: Tehran University of Medical Sciences; 2009.
- 33.Bharti SK, Krishnan S, Kumar A, Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther Adv Endocrinol Metab. 2018;9(3):81–100. doi: 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimabad MN, Niknia S, Golnabadi MB, Poor SF, Hajizadeh MR, Mahmoodi M. Effect of Citrullus colocynthis Extract on Glycated Hemoglobin Formation (In Vitro) Eurasian J Med. 2020;52(1):47–51. doi: 10.5152/eurasianjmed.2020.19223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benariba N, Djaziri R, Bellakhdar W, Belkacem N, Kadiata M, Malaisse WJ, et al. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extracts. Asian Pac J Trop Biomed. 2013;3(1):35–40. doi: 10.1016/S2221-1691(13)60020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javadzadeh HR, Davoudi A, Davoudi F, Valizadegan G, Goodarzi H, Mahmoodi S, et al. Citrullus colocynthis as the Cause of Acute Rectorrhagia. Case Rep Emerg Med. 2013;2013:652192. doi: 10.1155/2013/652192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savaj S, Ghaffari M, Abbasi MA, Azar J. Acute interstitial nephritis induced by Citrullus Colocynthis. Iran J Kidney Dis. 2017;11(5):385–7. [PubMed] [Google Scholar]
- 38.Qazan W, Almasad MM, Daradka H. Short and long effects of Citrullus colocynthis L. on reproductive system and fertility in female Spague-Dawley rats. Pak J Biol Sci. 2007;10(16):2699–703. doi: 10.3923/pjbs.2007.2699.2703. [DOI] [PubMed] [Google Scholar]
- 39.Al-Yahya MA, AH AL-F, Adam SE. Preliminary toxicity study on the individual and combined effects of Citrullus colocynthis and Nerium oleander in rats. Fitoterapia. 2000;71(4):385–91. doi: 10.1016/S0367-326X(00)00135-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 31 KB)