Abstract
Increasing ocean temperatures threaten coral reefs globally, but corals residing in habitats that experience high thermal variability are thought to be better adapted to survive climate-induced heat stress. Here, we used long-term ecological observations and in situ temperature data from Heron Island, southern Great Barrier Reef to investigate how temperature dynamics within various thermally variable vs. thermally stable reef habitats change during a marine heatwave and the resulting consequences for coral community survival. During the heatwave, thermally variable habitats experienced larger surges in daily mean and maxima temperatures compared to stable sites, including extreme hourly incursions up to 36.5 °C. The disproportionate increase in heat stress in variable habitats corresponded with greater subsequent declines in hard coral cover, including a three-times greater decline within the thermally variable Reef Flat (70%) and Deep Lagoon (83%) than within thermally stable habitats along sheltered and exposed areas of the reef slope (0.3–19%). Interestingly, the thermally variable Reef Crest experienced comparatively small declines (26%), avoiding the most severe tidal ponding and resultant heat stress likely due to proximity to the open ocean equating to lower seawater residence times, greater mixing, and/or increased flow. These results highlight that variable thermal regimes, and any acclimatization or adaptation to elevated temperatures that may lead to, do not necessarily equate to protection against bleaching and mortality during marine heatwaves. Instead, thermally stable habitats that have greater seawater exchange with the open ocean may offer the most protection to corals during the severe marine heatwaves that accompany a changing climate.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00338-022-02328-6.
Keywords: Environmental variability, Extreme environments, Climate change, Thermal stress, Coral bleaching, Coral reefs
Introduction
Coral reefs are among the most threatened ecosystems on Earth, with half of the world’s reef-building corals lost since 1950 (Eddy et al. 2021). While the decline of coral reefs is attributable to a range of stressors (De’ath et al. 2012), increasing ocean temperatures are currently considered among the greatest threats to reef survival (Hughes et al. 2018; Hoegh-Guldberg et al. 2019; Van Woesik et al. 2022). Increases in seawater temperature above a coral’s natural thermal threshold by as little as 1 °C can lead to the breakdown of the symbiosis between the coral host and endosymbiotic algal partners, a process known as coral bleaching (Hoegh-Guldberg 1999). Mass coral bleaching events are becoming more frequent and are now occurring several times a decade (Hughes et al. 2021). On the Great Barrier Reef (GBR), three recent marine heatwaves have occurred within a five-year period (2016, 2017 and 2020), resulting in greater levels of coral bleaching and mortality relative to prior bleaching events (e.g., 1998, 2002), likely due to more sustained heat stress and shorter recovery times in recent events (Berkelmans et al. 2004; Hughes et al. 2017) but see (DeCarlo and Harrison 2019). Remotely sensed temperature data and aerial surveys have resulted in a comprehensive description of the patterns of thermal stress and coral bleaching across the latitudinal length of the GBR during these events (Berkelmans et al. 2004; Hughes et al. 2017, 2021); however, the coarse spatial resolution (> 5 km) of these methods do not effectively capture reef-scale patterns in heat stress (Wall et al. 2015; Green et al. 2019), which can vary on the scale of meters and lead to substantial differences in bleaching and mortality within short (< 1 km) distances (Pineda et al. 2013; Schoepf et al. 2020).
Individual reef systems are made up of interconnected geomorphological zones that are characterized by distinct diel and seasonal fluctuations in multiple seawater physicochemical parameters (Phinn et al. 2012; Brown et al. 2018; Cyronak et al. 2020). For example, reef flats and lagoons are subject to semidiurnal tidal oscillations, resulting in extreme short-term variability in temperature, pH, irradiance, and oxygen, whereas conditions on neighboring subtidal fore reefs and reef slopes are typically more stable (Oliver and Palumbi 2011; Schoepf et al. 2020; Rathbone et al. 2022). As such, the physicochemical conditions to which reef communities are exposed can vary dramatically across small spatial scales (Cyronak et al. 2020; Reid et al. 2020; Kekuewa et al. 2021), often resulting in distinct benthic communities across habitats (Phinn et al. 2012; Brown et al. 2018; Roelfsema et al. 2021). These complexities mean that disentangling individual and interactive effects of seawater biogeochemistry constituents on coral thermal tolerance (i.e., the temperature at which corals bleach) and coral community resilience (i.e., the amount of bleaching and mortality experienced by a community during a heatwave) can be difficult. Despite this challenge, diel temperature variability has emerged as an important factor in promoting elevated coral bleaching thresholds (Oliver and Palumbi 2011; Kenkel et al. 2013; Schoepf et al. 2015, 2020), and thus has been predicted to promote coral survival in response to intensifying marine heatwaves (Safaie et al. 2018; Sully et al. 2019). Corals can currently tolerate extreme temperature conditions as a result of evolutionary selection over generations (i.e., adaptation) and/or non-genetic increases in fitness (i.e., acclimatization) through mechanisms such as phenotypic plasticity, harboring stress-tolerant symbiont communities, higher baseline expression of stress response genes, and/or epigenetics (Bay and Palumbi 2014; Palumbi et al. 2014; Kenkel and Matz 2016; Putnam 2021), providing a glimmer of hope that they will be able to acclimate and/or adapt to a changing climate. With the increasing frequency and severity of marine heatwaves, it has become critically important to understand how the additional heat accumulation associated with marine heatwaves affects coral communities when superimposed on top of thermally variable conditions in situ (Brown and Barott 2022). While experimental assessments from thermally variable vs. adjacent stable habitats have demonstrated elevated heat tolerance in corals from the variable habitats (Oliver and Palumbi 2011; Kenkel et al. 2013; Schoepf et al. 2015), we are only just beginning to observe the influence of climate change-induced heatwaves on these heat tolerant communities in the field. For example, recent studies on a handful of species have reported contrasting responses of corals from thermally variable habitats during in situ heatwaves, from severe coral bleaching (Schoepf et al. 2020; Ainsworth et al. 2021) and a reduction in thermal tolerance (Klepac and Barshis 2020) to rapid recovery (Schoepf et al. 2020), with differences likely due to the species examined and/or the duration and magnitude of heat stress sustained. As such, whether coral populations and communities acclimated and/or adapted to variable thermal conditions can resist bleaching during increasingly frequent and severe marine heatwaves remains an important outstanding question.
To test the hypothesis that thermally variable reef environments will become important spatial refugia for coral reefs in a changing climate, we evaluated changes in coral reef benthic community composition across five different geomorphological zones of Heron Reef in the aftermath of a severe marine heatwave that occurred in 2020 (Ainsworth et al. 2021; Hughes et al. 2021). By incorporating a range of common thermally stable (sheltered and exposed reef slopes) and thermally variable (reef crest, reef flat, shallow lagoon, and deep lagoon) geomorphological habitats, we were able to investigate the responses of benthic assemblages spanning diverse life history strategies (e.g., competitive, stress-tolerant), growth forms, and symbiont clade/subclades associations to the heatwave (Fig. 1). In addition, to better understand how temperature regimes and heat stress varied among the geomorphological zones, we recorded continuous in situ temperature profiles at high spatial resolution during two year-long periods that included an ambient (non-heatwave) time period (September 2015–August 2016) and a severe marine heatwave (September 2019–August 2020). Together, temperature data and benthic community composition from the aftermath of the 2020 heatwave were compared between thermally variable and stable sites (n = 8), and to data from previous non-bleaching years (July 2015 and August 2016) along identical transects at these same locations. This ecological approach is powerful as it allows us to investigate how a diversity of coral reef communities that have adapted or acclimated over their lifetime to distinct physicochemical regimes respond to heatwaves within their native habitat. Understanding coral responses to heatwaves within this complex, ecologically relevant context is critical for uncovering real world responses of coral communities to better predict their fate in the ongoing climate crisis.
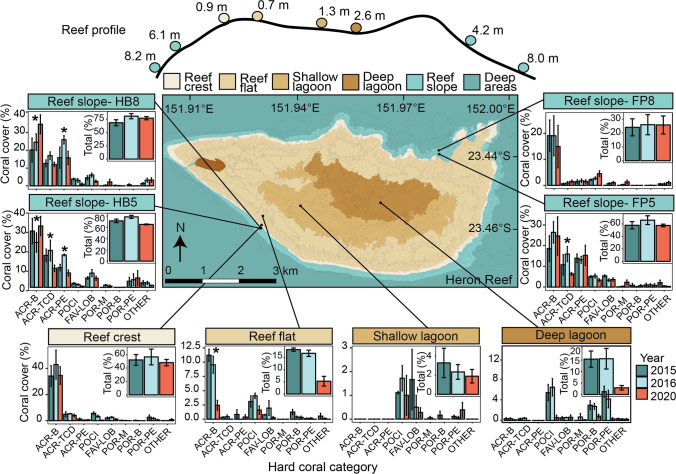
Fig. 1.
Hard coral abundance by site and year across Heron Reef. Cross section of Heron Reef indicating each site and mean depth (m). Map shading represents geomorphological habitats, redrawn from the data of Phinn et al. 2012. Hard coral cover is displayed by functional group, with inset showing total coral cover (% ± SE; n = 3). Asterisks (*) indicate where there are significant (p < 0.05) differences within categories. ACR-B, Acroporidae- branching; ARC-TCD, Acroporidae- tabular/corymbose/digitate; ACR-PE, Acroporidae- plating/encrusting; POCI, Pocilloporidae; FAV-LOB, Faviidae-Lobophyllidae; POR-M, Poritidae-massive; POR-B, Poritidae-branching; POR-PE, Poritidae-encrusting/plating varieties; and OTHER, other hard corals (including non-scleractinian corals)
Materials and methods
Study location
This study was conducted across eight sites at Heron Island, southern Great Barrier Reef (23°27′ S 151°55′ E), previously characterized in detail (Brown et al. 2018, 2021). Briefly, sites included each geomorphological habitat of Heron Reef: reef slope, reef crest, reef flat, shallow lagoon and deep lagoon (Phinn et al. 2012) (Fig. 1). The geomorphological habitats of Heron Reef are distinguished by diverse benthic communities, with hard coral cover higher within the reef slope and macroalgae cover greater within the lagoonal habitats (Brown et al. 2018; Roelfsema et al. 2021). Reef-wide coral cover in 2015 and 2016 was among the highest observations in the past 60 years (Connell et al. 1997; Brown et al. 2018; Roelfsema et al. 2021) (e.g., ~ 75% within the south-west reef slope and ~ 20% within lagoon), making these years optimal as a recent baseline record. Within the reef slope habitat, four sites were established: two within the north-east section of the reef (Fourth Point, 4.2 m and 8 m) and two within the south-west (Harry’s Bommie, 6.1 m and 8.2 m) (Fig. 1). The northeast of Heron Reef is the exposed side, subject to extreme wave forces during cyclones, whereas the south-west is sheltered from waves generated by both the south-east trade winds and extreme wave action of cyclones (Connell et al. 1997). One site was established in each other geomorphological habitat, with each site sharing its name: Reef Crest (RC; 0.9 m), Reef Flat (RF; 0.7 m), Shallow Lagoon (SL; 1.3 m) and Deep Lagoon (DL; 2.6 m) (Fig. 1). Inside the lagoon, semidiurnal tidal fluctuations result in higher variability in temperature and pH than reef slope sites (Brown et al. 2018; Cyronak et al. 2020) (Fig. 2, Fig. S1). Photosynthetically active radiation (PAR; µmol quanta m−2 s−1) is lower within reef slope habitats (HB5: 75.9, HB8: 72.8, FP5: 179.4, FP8: 58.9) than within the lagoon habitats (RC: 199.2, RF: 371.7, SL: 201.8, DL: 198.8), due to differences in depth (Brown et al. 2018; Cyronak et al. 2020). Mean depth and PAR were determined by use of Conductivity Temperature Depth (CTD) units that continuously recorded between July 2015–November 2016 (SBE 16plus V2 SEACAT fitted with an auxiliary PAR sensor, Satlantic/ECO-PAR sensor) (see Brown et al. 2018 for more detailed methodology).
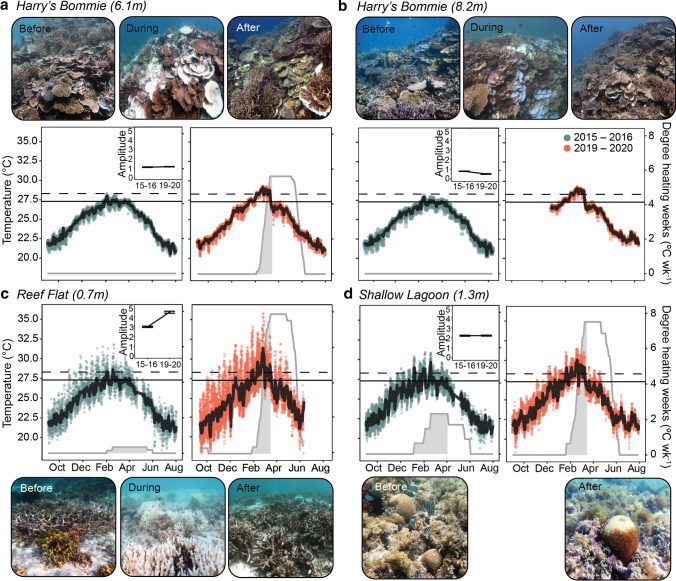
Fig. 2.
Temperature profiles and representative images from thermally stable and thermally variable habitats across Heron Reef. Images show coral communities before thermal stress (November 2015), during the peak of the marine heatwave (March 2020) and five months after the marine heatwave (August 2020). In situ temperature profiles and heat stress accumulation recorded from September 2015–August 2016 and September 2019–August 2020 at four sites across Heron Reef: (A) Thermally stable reef slope at 5 m depth (Harry’s Bommie 5 m), (B) thermally stable reef slope 8 m depth (Harry’s Bommie 8 m), (C) thermally variable reef flat and (D) thermally variable shallow lagoon. Points indicate hourly measurements and solid black lines indicate mean daily temperatures. Heat stress accumulation (gray shading) was estimated by degree heating weeks (solid gray line) calculated from mean daily temperatures. Degree heating weeks were not calculated for (B) in 2019–2020 due to an incomplete record. Solid horizontal line indicates the region’s climatological maximum monthly mean (MMM; 27.3 °C) and dashed horizontal line indicates the region’s coral bleaching threshold (MMM + 1 °C; 28.3 °C). Insets show mean daily temperature amplitude (°C ± SE). Image credit: Before, After: Kristen Brown; During: Aaron Chai
Benthic community composition
Benthic community composition was recorded in August 2020 using the method described by Brown et al. (2018). During each survey, three replicate 15 m transects were laid north, east, and west from a permanent reference point. Quadrats (0.25 m2) were alternated right and left every 0.5 m along the transect line, totalling 30 quadrats per transect. Percent cover of each quadrat was recorded in situ from 22 specific categories. The four main categories were hard coral, other invertebrates (including soft corals and sponges), algae and substrate. Hard corals were differentiated into family and growth form: Acroporidae- tabular/corymbose/digitate (ARC-TCD), Acroporidae- branching (ACR-BRA), Acroporidae- plating/encrusting (ACR-PE), Pocilloporidae (POCI); Poritidae-massive (POR-M); Poritidae-encrusting/plating varieties (POR-PE); Poritidae-branching (POR-BRA); Faviidae-Lobophyllidae (FAV-LOB); and other hard corals (including non-scleractinian corals). Bleaching status was recorded to family and growth form for all live corals and due to low bleaching prevalence across sites (Fig. S2), combined as ‘bleached hard coral’ for analyses. Macroalgae were separated as: fleshy macroalgae, calcifying algae of the genus Halimeda, articulate/crustose coralline algae (ACA/CCA) and turf algae/cyanobacteria. Substrate was divided into sand/sediment, coral rubble and recently dead hard coral (i.e., coral skeletons with epithetic algal community with turf height < 3 mm). Recently dead hard coral was also recorded to family and growth form where possible (Fig. S2). Benthic community composition in 2020 was compared to data collected in July 2015 and August 2016 along the same transects (Brown et al. 2018). All surveys were conducted within the same season (austral winter), because macroalgae display significant shifts in composition and abundance across seasons (Brown et al. 2018). The relative changes in hard coral (including bleached coral), algae and hard substrate (including coral rubble and recently dead coral) were determined by subtracting the mean between years and dividing by the initial cover:
Evaluation of thermal stress
Seawater temperatures were recorded at hourly intervals using cross-calibrated HOBO Pendant loggers (UA-001–64; ± 0.552 °C accuracy) between September 2019 and August 2020. Logger accuracy was assessed at the end of the deployment period using a water bath (Thermo Scientific Precision TSGP20). Due to logger failures, only a partial temperature record was obtained at HB8 (from December 2019), RC (from February 2020) and DL (from February 2020) and there is no temperature record at FP5 (Table 1, Fig. S1). No loggers were deployed at FP8 during 2019–2020. Seawater temperatures recorded in 2019–2020 were compared to temperatures recorded at the same locations in 2015–2016 (see Brown et al. 2018 for detailed methodology). The mean, maximum, minimum, and mean daily amplitude were calculated at each site for the two periods September 2015–August 2016 and September 2019–August 2020 (Table 1). Daily (24 h) mean temperatures (T) were extracted from the logger data. The climatological maximum monthly mean (MMM) of Heron Reef is 27.3 °C (Weeks et al. 2008). Temperature anomalies, or ‘hotspots’ (HS), were calculated from the logger data using U.S. National Oceanic and Atmospheric Administration (NOAA) Coral Reef Watch (CRW) methodology (Eakin et al. 2010); if T, representing the daily mean temperature, exceeded the region’s coral bleaching threshold (MMM + 1 °C; 28.3 °C), then the MMM was subtracted from T:
Table 1.
Temperature metrics across Heron Reef by site and year
| Period | Site | Mean | SE | Minimum | Maximum | Mean daily amplitude | SE | Degree heating weeks |
|---|---|---|---|---|---|---|---|---|
| Sept 2015–Aug 2016 | Harry's Bommie 5 m | 24.36 | 0.02 | 17.37 | 28.31 | 1.06 | 0.03 | 0.00 |
| Harry's Bommie 8 m | 24.56 | 0.02 | 19.75 | 28.25 | 0.81 | 0.02 | 0.00 | |
| Fourth Point 5 m | 24.44 | 0.02 | 16.34 | 28.60 | 1.27 | 0.03 | 0.00 | |
| Reef crest | 24.51 | 0.03 | 17.54 | 32.17 | 2.81 | 0.07 | 0.00 | |
| Reef flat | 23.97 | 0.03 | 18.04 | 31.80 | 2.96 | 0.06 | 2.85 | |
| Shallow lagoon | 24.91 | 0.03 | 18.74 | 30.92 | 2.22 | 0.05 | 2.26 | |
| Deep lagoon | 24.45 | 0.03 | 18.96 | 30.02 | 1.34 | 0.03 | 2.25 | |
| Sept 2019– Aug 2020 | Harry's Bommie 5 m | 24.85 | 0.02 | 20.04 | 29.35 | 1.11 | 0.02 | 5.60 |
| Harry's Bommie 8 m$ | 25.50 | 0.03 | 21.57 | 29.35 | 0.53 | 0.03 | ||
| Fourth Point 5 m | Temperature logger lost | |||||||
| Reef crest* | 24.22 | 0.04 | 17.48 | 33.22 | 2.43 | 0.08 | ||
| Reef flat | 25.32 | 0.03 | 18.05 | 36.49 | 4.45 | 0.10 | 7.84 | |
| Shallow lagoon | 24.85 | 0.03 | 18.33 | 31.47 | 2.23 | 0.05 | 7.51 | |
| Deep lagoon* | 26.06 | 0.04 | 22.62 | 30.05 | 1.21 | 0.05 | ||
Degree heating weeks were not calculated for sites where a complete temperature record was not obtained
* Data recorded from December 2019
$ Data recorded from February 2020
Importantly, we did not use nighttime-only temperatures, as is done with NOAA CRW, instead choosing to use 24 h mean temperatures due to the diel variability across sites. Thermal anomalies were then summed across a rolling 12-week (90-day) period to determine the extent of thermal stress in degree heating weeks (DHW; oCwk−1):
Statistical analyses
Differences in mean daily seawater temperatures between year (two levels: 2015–2016, 2019–2020) and site (three levels: HB5, RF, and SL) were explored using a linear model. Differences in benthic community composition were analyzed using permutational multivariate analysis of variance (PERMANOVA) and principal components analysis (PCA), with the fixed effects of site (eight levels: HB8, HB5, RC, RF, SL, DL, FP5, FP8) and year (three levels: 2015, 2016, 2020) using the adonis and rda functions in the vegan package, respectively (Oksanen et al. 2013). Significant effects were followed by pairwise comparisons using the pairwiseAdonis2 function in the pairwiseAdonis package (Arbizu 2020). Resemblance matrices were obtained using Bray–Curtis dissimilarity and 9999 permutations. All temperature metrics (Table 1) were assessed using the vif function in vegan, and meaningless metrics were removed if variance inflation exceeded 5. Temperature metrics showing significant correlations with PCA ordination were plotted as vectors using the envfit function in vegan.
The significant interaction between site and year was further explored on the percent cover of specific benthic categories using a linear model. The significance of fixed effects and their interactions was determined using an analysis of variance with a type III error structure using the Anova function in car package (Fox et al. 2012). Significant interactive effects were followed by pairwise comparison of estimate marginal means using the emmeans package with Tukey HSD adjusted p values (Lenth et al. 2018). Data were tested for homogeneity of variance and normality of distribution through graphical analyses of residual plots for all models. All statistical analyses were done using R version 4.0.0 software (R Core Team 2021), and graphical representations were produced using the package ggplot2 (Wickham 2016).
Results
Heat stress varied between habitats during the 2020 heatwave
Mean daily seawater temperatures were significantly hotter in September 2019–August 2020 when compared to September 2015–August 2016 across all sites (F = 4.03, p = 0.04) (Table 1, Fig. 2, Fig. S1, Table S1). Maximum temperatures recorded were between 0.55–4.69 °C warmer in 2019–2020 than 2015–2016 across all sites (Table 1, Fig. 2). Across the three sites where a complete temperature record was obtained in 2019–2020 (Harry’s Bommie–5 m depth, Reef Flat and Shallow Lagoon), hourly temperatures were significantly influenced by the interaction of year and site (F = 161.92, p < 0.0001). Pairwise comparisons revealed that within the thermally variable Reef Flat, annual mean temperatures were nearly one and a half degrees warmer (1.35 °C) in 2019–2020 when compared to 2015–2016 (p < 0.0001), whereas the stable reef slope site (Harry’s Bommie 5 m) only exhibited an increase of 0.49 °C (Table 1, Fig. 2). During the heatwave, the mean daily amplitude increased at the Reef Flat, where it was 1.49 °C greater in 2020 than in 2015–2016 (4.45 °C vs. 2.96 °C, respectively) (Table 1, Fig. 2). In contrast, the mean daily amplitude did not change within the thermally stable reef slope during the heatwave (1.11 °C vs. 1.06 °C). Increased variability within the Reef Flat was driven by extreme hourly temperatures of up to 36.49 °C, which coincided with midday low tides in mid-February during the heatwave (Fig. 2). As a result, the accumulation of heat stress, measured as degree heating weeks (DHW), was greatest within the thermally variable Shallow Lagoon and Reef Flat during the 2020 heatwave, reaching a maximum of 7.51 °C wk−1 and 7.84 °C wk−1, respectively (Table 1, Fig. 2). In contrast, hourly temperature maxima within thermally stable reef slope sites never exceeded 30 °C (maximum of 29.35 °C), and Harry’s Bommie 5 m only reached a maximum of 5.60 °C wk−1 during the 2020 heatwave (Table 1, Fig. 2).
Benthic community composition shifted following the marine heatwave
Due to the COVID-19 pandemic and associated travel restrictions, we were unable to quantitatively assess coral bleaching during the peak of heat stress in March 2020; however, we were able to confirm severe and extensive coral bleaching within the thermally variable Reef Flat and selective coral bleaching within the thermally stable reef slope (Harry’s Bommie) from photographs taken at that time (Fig. 2, Fig. S3–S4). Severe and extensive coral bleaching was observed within the thermally variable Reef Flat during the heatwave (Fig. 2, Fig. S3), with Ainsworth et al. 2021 reporting up to 60% of corals experiencing bleaching by mid-February 2020. In contrast, Ainsworth et al. 2021 observed that only 10% of corals within the thermally stable reef slope were experiencing bleaching at the same time, which was in agreement with our observations (Fig. 2, Fig. S4). We found that branching Acropora, the most abundant hard coral across Heron Reef (Brown et al. 2018), exhibited differential patterns in the susceptibility between the thermally stable and variable habitats (Fig. 2, Fig. S3–S4). Ainsworth et al. 2021 further supported these findings, reporting that symbiont densities were 50-fold less in branching Acropora from the reef flat than corals impacted by bleaching on the coral reef slope at the peak of the heatwave (Ainsworth et al. 2021). Within thermally stable reefs, we observed that foliose and plating Montipora and tabular, corymbose, and digitate Acropora that were the most susceptible to bleaching (Fig. 2, Fig. S4).
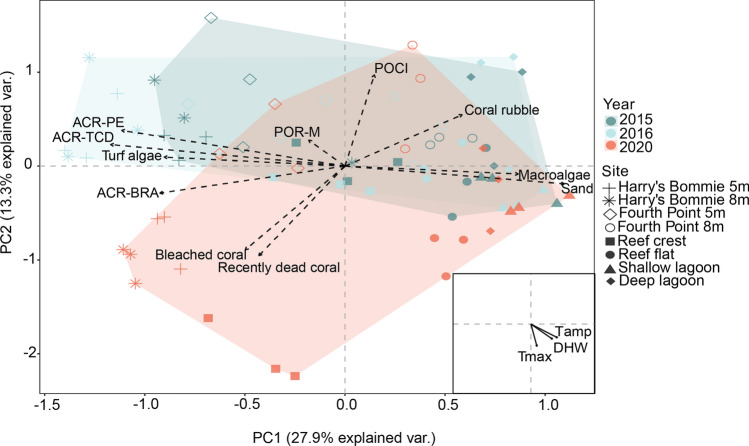
PERMANOVA testing revealed that benthic community composition was significantly influenced by site (pseudo F = 27.34, p < 0.0001) and year (pseudo F = 13.49, p < 0.0001) (Table S2). Principal component analysis further revealed distinct patterns through time, with the first two principal component (PC) axes explaining 27.9% and 13.3% of the variance, respectively. PC1 was most closely correlated with Pocilloporidae (POCI) and coral rubble, and PC2 with Acroporidae- tabular/corymbose/digitate (ARC-TCD), Acroporidae- branching (ACR-BRA) and Acroporidae- plating/encrusting (ACR-PE) (Fig. 3, Table S4). Convex hulls revealed significant overlap between non-heatwave years (2015 and 2016), with 2020 shifting into space distinguished by bleached hard coral and recently dead coral. Visual patterns reflected significant differences between years, with pairwise comparisons revealing no significant differences in benthic communities between 2015 and 2016 (p = 0.79), yet significant and nearly significant differences between 2015 and 2020 (p = 0.04) and 2016 and 2020 (p = 0.06), respectively (Fig. 3, Table S3). Between sites, significant overlap was observed between three of the four thermally stable reef slope sites: Fourth Point 5 m, Harry’s Bommie 5 m, and Harry’s Bommie 8 m. Benthic composition of thermally stable Fourth Point 8 m, having suffered severe cyclone damage ~ 10 years earlier (March 2009) (Brown et al. 2021), was distinct from the other reef slope sites and was closely aligned to lagoonal sites (Fig. 3). The thermally variable Reef Flat, Shallow Lagoon and Deep Lagoon sites grouped in neighboring space, and most closely aligned with communities with greater coral rubble, macroalgae and sand (Fig. 3). Maximum temperature (r2 = 0.62, p = 0.0001), daily temperature amplitude (r2 = 0.49, p = 0.0001) and DHW (r2 = 0.45, p = 0.0001) all were significant predictors of benthic community composition, while annual mean temperature was not significant (r2 = 0.04, p = 0.35) (Fig. 3, Table S5).
Fig. 3.
Principal components analysis (PCA) of benthic community composition by year. Convex hulls represent year groupings. Biplot vectors represent key benthic categories (dash-line arrows). Inset displays significant (p = 0.0001) temperature metrics (black arrows) according to squared correlation coefficients (r2). ARC-TCD, Acroporidae- tabular/corymbose/digitate; ACR-BRA, Acroporidae- branching; ACR-PE, Acroporidae- plating/encrusting; POCI, Pocilloporidae; POR-MASS, Poritidae-massive; DHW, Degree heating weeks; Tamp, Diel temperature amplitude; Tmax, Maximum temperature
Across all of Heron Reef, total hard coral cover significantly decreased from 42.9% cover in 2016 to 35.5% cover in 2020 (decrease of 7.6 ± 2.1%) (F = 3.79, p = 0.001) (Fig. S5, Table S6). Some remnant coral bleaching (up to 3% cover) was still observed five months after peak heat stress (Fig. 2, Fig. S2). There were no significant differences in the abundance of algae or other benthic invertebrates between years (p = 0.34) (Fig. S5). Also, there was no significant change in hard substrate cover between 2015 and 2016; however, between 2016 and 2020, there was a significant increase in hard substrate cover (9.56 ± 2.1%) (p < 0.001), driven by an increase in recently dead coral and coral rubble (Fig. S2, S5).
Decline in hard coral cover was greatest within thermally variable habitats
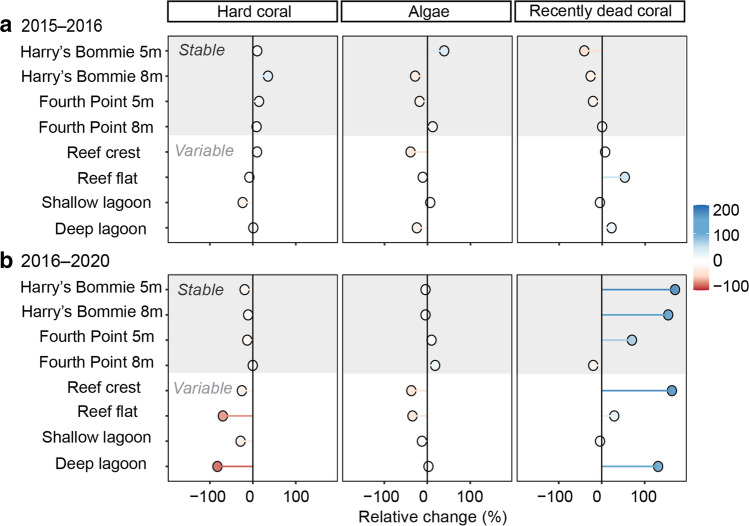
The largest relative declines in coral cover from 2016 to 2020 were observed within the thermally variable habitats of the Reef Crest (− 25.6%), Reef Flat (− 69.8%), Deep Lagoon (− 82.6%), and Shallow Lagoon (− 28.6%) (Fig. 4, Table S8), the latter of which historically has had only ~ 3% coral cover (Brown et al. 2018). At the thermally stable reef slope sites, declines occurred but were of lesser magnitude. For example, on the south-west reef slope at Harry’s Bommie 5 m, a relative decline of − 19.1% was observed, whereas at 8 m coral cover declined by − 10.9% (Fig. 4, Table S8). On the north-east reef slope at Fourth Point, the 8 m site experienced virtually no relative change in coral cover (− 0.26%), whereas at 5 m, coral cover declined by − 13.0% (Fig. 4, Table S8). Within the stable sites, macroalgae cover increased by + 9.9% and + 18.4% on the north-east reef slope at 5 m and 8 m, respectively, whereas on the south-west reef slope, macroalgae cover decreased by − 4.0% at both 5 m and 8 m (Fig. 4, Table S8). The highly variable habitats of the Reef Flat, Shallow Lagoon, and Deep Lagoon experienced − 34.5%, − 12.4% and + 2.8% changes in macroalgal cover, respectively (Fig. 4, Table S8). The Reef Crest experienced the largest relative declines in macroalgae (− 37.2%) (Fig. 4, Table S8). Hard substrate, including bare substrate, recently dead coral, and coral rubble, increased across most sites between 2016–2020 from 29.2% and up to 170.8% (Fig. 4, Table S8). All absolute and relative changes in hard coral, algae and hard substrate are presented in Tables S7 and S8.
Fig. 4.
Relative change in benthic cover across thermally stable and variable sites. (A) Relative change (%) in cover between 2015 and 2016. (B) Relative change in cover between 2016 and 2020. Benthic categories are displayed at the top of the Figure: hard coral (includes bleached coral); algae; and recently dead coral (includes coral rubble)
Changes in hard coral cover by functional group were habitat-specific
The abundance of ACR-TCD and ACR-PE significantly declined between 2016 and 2020 at the 5 m south-west reef slope site, by − 9.6 ± 4.6% and − 9.6 ± 2.1%, respectively (χ2 = 426.95, p < 0.001) (Fig. 1, Table S9). At the 8 m south-west reef slope site, there was also a significant reduction in ACR-PE (− 10.2 ± 4.1%) between 2016 and 2020 (Fig. 1). In contrast, ACR-BRA increased significantly between 2016 and 2020 at both the 5 m (+ 8.8 ± 4.1%) and 8 m (+ 10.1 ± 9.9%) south-west reef slope sites (Fig. 1). An increase in recently dead coral was also observed at the 5 m (+ 21.2 ± 2.3%) and 8 m (+ 16.5 ± 1.6%) south-west reef slope sites between 2016 and 2020 (Fig. 1). At the 5 m north-east reef slope, a significant decline in ACR-TCD (-9.5 ± 3.3%) and increase in recently dead coral (+ 9.4 ± 1.8%) was observed between 2016 and 2020 (Fig. 1). No significant changes were observed at 8 m on the north-east reef slope between years. Within the thermally variable Reef Flat, the abundance of ACR-BRA significantly declined between 2016 and 2020 (− 7.1 ± 2.2%), and recently dead coral increased (+ 19.9 ± 3.3%) (Fig. 1). Within the Deep Lagoon, there were notable, although nonsignificant reductions in POCI (− 5.9 ± 1.5%), Poritidae-branching (POR-BRA; − 1.9 ± 0.9%) and Poritidae-massive (POR-M; -3.9 ± 1.7%) and a significant increase in coral rubble between 2016 and 2020 (+ 10.3 ± 10.1%) (Fig. 1). No significant changes were observed at the Shallow Lagoon between years.
Discussion
Thermally variable habitats that currently endure extreme temperatures are predicted to promote heat-tolerant corals through both acclimatization and adaptation (Bay and Palumbi 2014; Palumbi et al. 2014; Kenkel and Matz 2016). Yet, whether these gains in heat tolerance by coral communities native to naturally variable environments increases preparedness and ultimately survival through extreme marine heatwaves remains a critical outstanding question. Here, we found that corals residing within thermally variable habitats that are able to withstand daily short-term, extreme fluctuations in temperatures are still highly sensitive to bleaching and mortality during climate change-driven heatwaves. We reveal that during the 2020 heatwave at Heron Reef, thermally variable reef sites experienced disproportionately greater increases in temperature relative to stable reef sites. This was driven by amplified daily temperature maxima (up to 36.5 °C at variable sites vs. 29.4 °C at stable sites), increases in the daily mean temperature (up to + 1.4 °C at variable sites vs. + 0.9 °C at stable sites), and greater increases in the magnitude of diel variability (up to ± 4.5 °C d−1 at variable sites vs. ± 1.1 °C d−1 at stable sites). This led to higher cumulative heat stress at variable (7.51–7.84 °C week−1) compared to stable sites (5.60 °C week−1)—a more pronounced increase in heat stress than if the heatwave had increased temperatures by the same amount across all habitats. While it is possible that thermal variability led to elevated thermal tolerance (i.e., higher bleaching threshold), this disproportionately higher heat stress appears to have negated any potential advantage of corals native to variable temperature regimes may have gained relative to corals in stable temperature regimes, leading to declines in hard coral cover that were three times greater in the thermally variable habitats than declines within thermally stable habitats (26– 82% vs. 0.3–19%, respectively). This difference in the additional heat stress and resultant mortality caused by an in situ heatwave in variable habitats representing four distinct geomorphological zones is notable, as static experimental heat stress assays would not have captured this natural environmental heterogeneity (Ziegler et al. 2021).
While temperature regimes that expose corals to sub-bleaching stress have been recognized as a mechanism to increase the physiological preparation for marine heatwaves events (Ainsworth et al. 2016; Safaie et al. 2018; Sully et al. 2019), this protective mechanism is expected to be lost as climate change intensifies (Schoepf et al. 2015; Ainsworth et al. 2016). Indeed, in our study, anomalously high temperatures within thermally variable habitats seemed to overwhelm the ability of corals to cope with heat stress, despite prior protective, sub-bleaching stress exposure. Temperature maxima within the thermally variable Reef Flat coincided with midday low tides when solar heating combined with tidal ponding to drive temperatures (recorded at five-minute intervals) briefly over 38 °C at the peak of the heatwave (mid-February 2020) (Ainsworth et al. 2021). Even hourly temperature maxima were ~ 5 °C hotter within the Reef Flat than temperatures previously experienced in a non-heatwave year. Extreme temperatures pushed diurnal variability above ± 4.4 °C d−1, levels which have been previously shown to lower heat tolerance (Schoepf et al. 2019). In contrast, corals from thermally stable reef slope habitats were exposed to the same, minimal diurnal variability (± 0.5–1.1 °C d−1) and temperature maxima only 1 °C hotter than temperatures from a non-heatwave year. While co-occurring environmental conditions (e.g., pCO2, oxygen, and flow) were not directly quantified, presumably higher irradiance due to shallower depths as well as decreased oxygen, elevated pCO2, and limited flow as a result of longer seawater residence times (Brown et al. 2018; Cyronak et al. 2020; Reid et al. 2020; Rathbone et al. 2022) could have exacerbated stress in corals from the inner lagoonal habitats (i.e., Reef Flat, Deep Lagoon). Surprisingly, the thermally variable Reef Crest demonstrated divergent patterns to the other thermally variable sites. Similar to the reef slope habitats, temperature maxima were only 1 °C hotter than temperatures from a non-heatwave year (32.2 °C in 2015 vs. 33.2 °C in 2020) and diurnal variability was nearly the same between heatwave (± 2.43 °C d−1) and non-heatwave years (± 2.96 °C d−1). It is important to note that a complete temperature record (i.e., from September 2019–August 2020) was not obtained at the Reef Crest, but we did capture the peak of the heatwave (February–August 2020) (Fig. S1). Even so, because irradiance (PAR; µmol quanta m−2 s−1) was similar between the Shallow Lagoon (201), Deep Lagoon (199) and Reef Crest (199) (Brown et al. 2018), we hypothesize that greater exposure to the open ocean resulted in greater mixing and flow (Reid et al. 2020; Kekuewa et al. 2021) — factors which are known to offer some protection to corals during marine heatwaves (Wall et al. 2015). Using the reef as a natural laboratory allowed us to capture these important differences in community resilience, which are not possible to predict and thus analogously replicate in experimental settings; however, future studies could investigate the thermal tolerance of corals across these locations using a controlled heat stress assay to better understand if thermal variability results in elevated heat tolerance under identical environmental conditions e.g., (Voolstra et al. 2020). Additionally, the effects of co-occurring environmental parameters that exacerbate or alleviate thermal stress both in situ and in controlled experimental settings are poorly understood and an important avenue of future studies (Ziegler et al. 2021; Van Woesik et al. 2022).
Differences in heat stress between habitats resulted in divergent patterns of coral community resilience in the aftermath of the marine heatwave. In 2015, 2018, and 2019, no marine heatwaves were recorded across the GBR, whereas in 2016, 2017 and 2020, significant marine heatwaves occurred (Hughes et al. 2021). In 2016 and 2017, aerial surveys revealed that Heron Reef experienced minimal coral bleaching at the peak of heat stress (2016: < 1% of corals, 2017: 1–10% of corals) (Hughes et al. 2017). Interestingly, in a study that examined patterns in coral cover across Heron Reef from 2002 to 2018, a notable reef-wide decline in hard coral cover (− 7.8%) was observed between 2015 and 2018, suggesting a more significant effect of the 2016 and 2017 marine heatwaves than implied from aerial bleaching surveys. While our study cannot determine the exact amount of coral cover lost during each heatwave event between 2016 and 2020, the data of Roelfsema et al. 2021 agree with our observations—specifically, that habitats that historically experienced the greatest thermal variability (i.e., Reef Flat) underwent the greatest declines in hard coral cover (Roelfsema et al. 2021). In our study, we expand on the observations of Roelfsema et al. 2021, incorporating five additional sites and long-term temperature data that demonstrates extreme temperature incursions during the heatwave were detrimental for corals inhabiting thermally variable habitats. Specifically, we reveal that the thermally variable Reef Flat and Deep Lagoon, which boasted ~ 18% coral cover in 2015 (Brown et al. 2018), were reduced to < 5% coral cover following the 2020 heatwave. These declines will have long-term impacts until populations can recover, which historically can take up to decades for populations faced with chronic disturbances (Connell et al. 1997), and in the interim, will likely drive reef accretion negative across > 50% of the Heron Reef platform (Brown et al. 2021). Again, the Reef Crest was different from other thermally variable sites, experiencing a comparatively small decline in coral cover (from 53% in 2015 to 48% in 2020), supporting the findings of others that habitats closer to the open ocean may experience some mitigation of the most severe tidal ponding and resultant heat stress (Reid et al. 2020).
The most significant species-specific declines in coral cover reflected patterns in bleaching susceptibility, with a loss of nearly all branching Acropora within the Reef Flat. Interestingly, within the thermally variable Reef Crest and across the thermally stable south-west reef slope, branching Acropora cover did not decline and even increased through time. There were no clear ‘winners’ within the thermally variable habitats, although some massive faviids experienced only partial mortality on the upper portion of the colonies (Fig. 2), and solitary polyp corals (e.g., Fungia and Heliofungia) seemed to experience negligible mortality. The contrasting patterns in the susceptibility of branching Acropora between the thermally stable and variable habitats observed here may be due to local adaption and/or cryptic species diversity between habitats (Kenkel and Matz 2016; Schoepf et al. 2020). Interestingly, our findings are in contrast to a recent study in western Australia that found that corals (principally branching acroporids) in more stable habitats suffered the most extensive mortality during a heatwave, while corals in more thermally variable habitats were able to fully recover within six months following coral bleaching (Schoepf et al. 2020). Differences between this study and Schoepf et al. (Schoepf et al. 2020) are likely due to the duration and magnitude of heat stress sustained. In our study, heat stress within the thermally variable habitat exceeded 7.8 °C wk−1, whereas in Schoepf et al. (Schoepf et al. 2020), heat stress peaked at ~ 4 °C wk−1 within the thermally variable intertidal habitat (Le Nohaïc et al. 2017). Taken together, corals inhabiting thermally variable environments may be able to withstand moderate heat stress events (e.g., < 8 °C wk−1); however, as ocean temperatures continue to rise, thermal protection gained from acclimatization may no longer protect corals from future marine heatwaves.
Conclusions
Short-term fluctuations in extreme temperatures have been suggested to reduce the risk of coral bleaching (Safaie et al. 2018; Sully et al. 2019) and promote coral heat tolerance (Oliver and Palumbi 2011; Palumbi et al. 2014; Schoepf et al. 2015, 2020). This study makes the critically important step of identifying that thermal protection gained from acclimatization to variable temperature regimes may not protect corals against bleaching and mortality during severe heatwaves due to disproportionate increases in temperatures in these habitats. Instead, deeper, thermally stable habitats and shallow habitats with greater flow are able to offer some protection from the most dangerous temperatures associated with marine heatwaves. As the climate continues to change, this study clearly shows that long-term monitoring is crucial to understand: (i) how thermal variability deviates during heatwaves within and between reef systems, and (ii) the ecological and physiological responses of individual organisms and benthic communities to thermal stress across multiple habitats. More research is needed to understand the interactions between various environmental conditions that co-occur within thermally variable habitats (e.g., irradiance, oxygen) and how multiple stressors influence coral survivorship. Nevertheless, urgent and collective efforts to limit climate-induced temperature increases to 1.5 °C remain critical to limit the loss of coral reef ecosystems now and into the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Aaron Chai for use of photographs, Adriana Campili for support in the field, and the staff of Heron Island Research Station.
Funding
This work was supported by National Geographic Society Early Career Grant EC-56459R-19 to KTB, the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement no. 796025 to GE, National Science Foundation (NSF) OCE award 1923743 to KLB, and the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies CE140100020 to SGD.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W. Climate change disables coral bleaching protection on the great barrier reef. Science. 2016;352:338–342. doi: 10.1126/science.aac7125. [DOI] [PubMed] [Google Scholar]
- Ainsworth TD, Leggat W, Silliman BR, Lantz CA, Bergman JL, Fordyce AJ, Page CE, Renzi JJ, Morton J, Eakin CM, Heron SF (2021) Rebuilding relationships on coral reefs: coral bleaching knowledge‐sharing to aid adaptation planning for reef users: Bleaching emergence on reefs demonstrates the need to consider reef scale and accessibility when preparing for, and responding to, coral bleaching. Bioessays 43(9):2100048 [DOI] [PubMed]
- Arbizu PM (2020) pairwiseAdonis: Pairwise multilevel comparison using adonis. https://github.com/pmartinezarbizu/pairwiseAdonis
- Bay RA, Palumbi SR. Multilocus adaptation associated with heat resistance in reef-building corals. Curr Biol. 2014;24:2952–2956. doi: 10.1016/j.cub.2014.10.044. [DOI] [PubMed] [Google Scholar]
- Berkelmans R, De’ath G, Kininmonth S, Skirving WJ. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions. Coral Reefs. 2004;23:74–83. doi: 10.1007/s00338-003-0353-y. [DOI] [Google Scholar]
- Brown KT, Barott KL (2022) The costs and benefits of environmental memory for reef-building corals coping with recurring marine heatwaves. Integr Comp Biol icac074. 10.1093/icb/icac074 [DOI] [PubMed]
- Brown KT, Bender-Champ D, Achlatis M, van Der Zande RM, Kubicek A, Martin SB, Castro-Sanguino C, Dove SG, Hoegh-Guldberg O. Habitat-specific biogenic production and erosion influences net framework and sediment coral reef carbonate budgets. Limnol Oceanogr. 2021;66:349–365. doi: 10.1002/lno.11609. [DOI] [Google Scholar]
- Brown KT, Bender-Champ D, Kubicek A, van der Zande R, Achlatis M, Hoegh-Guldberg O, Dove SG. The dynamics of coral-algal interactions in space and time on the southern great barrier reef. Front Mar Sci. 2018;5:181. doi: 10.3389/fmars.2018.00181. [DOI] [Google Scholar]
- Connell JH, Hughes TP, Wallace CC. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr. 1997;67:461–488. doi: 10.1890/0012-9615(1997)067[0461:AYSOCA]2.0.CO;2. [DOI] [Google Scholar]
- Cyronak T, Takeshita Y, Courtney TA, DeCarlo EH, Eyre BD, Kline DI, Martz T, Page H, Price NN, Smith J. Diel temperature and pH variability scale with depth across diverse coral reef habitats. Limnol Oceanogr Lett. 2020;5:193–203. doi: 10.1002/lol2.10129. [DOI] [Google Scholar]
- De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc National Acad Sci. 2012;109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo TM, Harrison HB. An enigmatic decoupling between heat stress and coral bleaching on the great barrier reef. PeerJ. 2019;7:e7473. doi: 10.7717/peerj.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L, Baca B, Bartels E, Bastidas C, Bouchon C. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS ONE. 2010;5:e13969. doi: 10.1371/journal.pone.0013969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy TD, Lam VWY, Reygondeau G, Cisneros-Montemayor AM, Greer K, Palomares MLD, Bruno JF, Ota Y, Cheung WWL. Global decline in capacity of coral reefs to provide ecosystem services. One Earth. 2021;4:1278–1285. doi: 10.1016/j.oneear.2021.08.016. [DOI] [Google Scholar]
- Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S. Package “car”. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Green RH, Lowe RJ, Buckley ML, Foster T, Gilmour JP (2019) Physical mechanisms influencing localized patterns of temperature variability and coral bleaching within a system of reef atolls. Coral Reefs
- Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res. 1999;50:839–866. [Google Scholar]
- Hoegh-Guldberg O, Jacob D, Taylor M, Guillén Bolaños T, Bindi M, Brown S, Camilloni IA, Diedhiou A, Djalante R, Ebi K, Engelbrecht F, Guiot J, Hijioka Y, Mehrotra S, Hope CW, Payne AJ, Pörtner H-O, Seneviratne SI, Thomas A, Warren R, Zhou G. The human imperative of stabilizing global climate change at 1.5°C. Science. 2019;365:eaaw6974. doi: 10.1126/science.aaw6974. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359:80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Kerry JT, Connolly SR, Álvarez-Romero JG, Eakin CM, Heron SF, Gonzalez MA, Moneghetti J. Emergent properties in the responses of tropical corals to recurrent climate extremes. Curr Biol. 2021;31:5393–5399.e3. doi: 10.1016/j.cub.2021.10.046. [DOI] [PubMed] [Google Scholar]
- Kekuewa SAH, Courtney TA, Cyronak T, Kindeberg T, Eyre BD, Stoltenberg L, Andersson AJ. Temporal and spatial variabilities of chemical and physical parameters on the Heron Island coral reef platform. Aquat Geochem. 2021;27:241–268. doi: 10.1007/s10498-021-09400-7. [DOI] [Google Scholar]
- Kenkel CD, Matz MV. Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat Ecol Evol. 2016;1:14. doi: 10.1038/s41559-016-0014. [DOI] [PubMed] [Google Scholar]
- Kenkel CD, Meyer E, Matz MV. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol Ecol. 2013;22:4322–4334. doi: 10.1111/mec.12390. [DOI] [PubMed] [Google Scholar]
- Klepac CN, Barshis DJ. Reduced thermal tolerance of massive coral species in a highly variable environment. Proc R Soc B. 2020;287:20201379. doi: 10.1098/rspb.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-14794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M. Emmeans: Estimated marginal means, aka least-squares means. R Package Version. 2018;1:3. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. Package “vegan”. Commun Ecol Package Ver. 2013;2:1–295. [Google Scholar]
- Oliver TA, Palumbi SR. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs. 2011;30:429–440. doi: 10.1007/s00338-011-0721-y. [DOI] [Google Scholar]
- Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. Mechanisms of reef coral resistance to future climate change. Science. 2014;344:895–898. doi: 10.1126/science.1251336. [DOI] [PubMed] [Google Scholar]
- Phinn SR, Roelfsema CM, Mumby PJ. Multi-scale, object-based image analysis for mapping geomorphic and ecological zones on coral reefs. Int J Remote Sens. 2012;33:3768–3797. doi: 10.1080/01431161.2011.633122. [DOI] [Google Scholar]
- Pineda J, Starczak V, Tarrant A, Blythe J, Davis K, Farrar T, Berumen M, da Silva JCB. Two spatial scales in a bleaching event: Corals from the mildest and the most extreme thermal environments escape mortality. Limnol Oceanogr. 2013;58:1531–1545. doi: 10.4319/lo.2013.58.5.1531. [DOI] [Google Scholar]
- Putnam HM (2021) Avenues of reef-building coral acclimatization in response to rapid environmental change. J Exp Biol 224(Suppl_1):jeb239319 [DOI] [PubMed]
- Rathbone M, Brown KT, Dove S. Tolerance to a highly variable environment does not infer resilience to future ocean warming and acidification in a branching coral. Limnol Oceanogr. 2022;67:272–284. doi: 10.1002/lno.11991. [DOI] [Google Scholar]
- R Core Team (2021) R: A language and environment for statistical computing. https://www.R-project.org/
- Reid EC, Lentz SJ, DeCarlo TM, Cohen AL, Davis KA (2020) Physical processes determine spatial structure in water temperature and residence time on a wide reef flat. J Geophys Res C: Oceans 125(12):e2020JC016543
- Roelfsema C, Kovacs EM, Markey K, Vercelloni J, Rodriguez-Ramirez A, Lopez-Marcano S, Gonzalez-Rivero M, Hoegh-Guldberg O, Phinn SR (2021) Benthic and coral reef community field data for Heron Reef, Southern Great Barrier Reef, Australia, 2002–2018. Sci Data 8(1):1–7 [DOI] [PMC free article] [PubMed]
- Safaie A, Silbiger NJ, McClanahan TR, Pawlak G, Barshis DJ, Hench JL, Rogers JS, Williams GJ, Davis KA. High frequency temperature variability reduces the risk of coral bleaching. Nat Commun. 2018;9:1671. doi: 10.1038/s41467-018-04074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepf V, Carrion SA, Pfeifer SM, Naugle M, Dugal L, Bruyn J, McCulloch MT. Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat Commun. 2019;10:1–10. doi: 10.1038/s41467-019-12065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepf V, Jung MU, McCulloch MT, White NE, Stat M, Thomas L. Thermally variable, macrotidal reef habitats promote rapid recovery from mass coral bleaching. Front Mar Sci. 2020;7:245. doi: 10.3389/fmars.2020.00245. [DOI] [Google Scholar]
- Schoepf V, Stat M, Falter JL, McCulloch MT. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci Rep. 2015;5:1–14. doi: 10.1038/srep17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully S, Burkepile DE, Donovan MK, Hodgson G, Van Woesik R. A global analysis of coral bleaching over the past two decades. Nat Commun. 2019;10:1–5. doi: 10.1038/s41467-019-09238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra CR, Buitrago-López C, Perna G, Cárdenas A, Hume BCC, Rädecker N, Barshis DJ. Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob Chang Biol. 2020;26:4328–4343. doi: 10.1111/gcb.15148. [DOI] [PubMed] [Google Scholar]
- Wall M, Putchim L, Schmidt GM, Jantzen C, Khokiattiwong S, Richter C. Large-amplitude internal waves benefit corals during thermal stress. Proc Biol Sci. 2015;282:20140650. doi: 10.1098/rspb.2014.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks SJ, Anthony KRN, Bakun A, Feldman GC, Guldberg OH. Improved predictions of coral bleaching using seasonal baselines and higher spatial resolution. Limnol Oceanogr. 2008;53:1369–1375. doi: 10.4319/lo.2008.53.4.1369. [DOI] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. Springer; 2016. [Google Scholar]
- Van Woesik R, Shlesinger T, Grottoli AG, Toonen RJ, Vega Thurber R, Warner ME, Marie Hulver A, Chapron L, McLachlan RH, Albright R, Crandall E, DeCarlo TM, Donovan MK, Eirin-Lopez J, Harrison HB, Heron SF, Huang D, Humanes A, Krueger T, Madin JS, Manzello D, McManus LC, Matz M, Muller EM, Rodriguez-Lanetty M, Vega-Rodriguez M, Voolstra CR, Zaneveld J (2022) Coral-bleaching responses to climate change across biological scales. Glob Chang Biol 28(14):4229–4250 [DOI] [PMC free article] [PubMed]
- Ziegler M, Anton A, Klein SG, Rädecker N, Geraldi NR, Schmidt-Roach S, Saderne V, Mumby PJ, Cziesielski MJ, Martin C, Frölicher TL, Pandolfi JM, Suggett DJ, Aranda M, Duarte CM, Voolstra CR. Integrating environmental variability to broaden the research on coral responses to future ocean conditions. Glob Chang Biol. 2021;27:5532–5546. doi: 10.1111/gcb.15840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.