Abstract
Purpose:
To report retinal nerve fiber layer thickness (RNFLT) in eyes with amblyopia compared with contralateral healthy eyes.
Methods:
In this cross-sectional study, we included patients with anisometropic amblyopia, strabismic amblyopia, and mixed amblyopia. All subjects underwent complete ophthalmic examination, including RNFLT measurement with time-domain OCT (Stratus OCT) and scanning laser polarimeter (GDX VCC). A paired “t” test was used to compare average and quadrant-wise RNFL thickness between the amblyopic and contralateral normal eyes. In addition, an analysis of variance test was used to compare various RNFL thickness parameters between the three groups.
Results:
A total of 33 eyes of 33 subjects with anisometropic amblyopia, 20 eyes of 20 subjects with strabismic amblyopia, and 38 eyes of 38 subjects with mixed amblyopia were included. In the anisometropic amblyopia group, the average RNFLT in the amblyopic eye was 98.2 mm and 99.8 mm in the fellow normal eye (P = 0.5), the total foveal thickness was 152.82 mm (26.78) in the anisometropic eye and 150.42 mm (23.84) in the fellow eye (P = 0.38). The difference between amblyopic and contralateral normal eye for RNFL and macular parameters was statistically insignificant in all three groups. The RNFL thickness in four quadrants was similar in the amblyopic and non-amblyopic eye between all three groups and statistically non-significant.
Conclusion:
Our study showed that RNFL thickness was similar in amblyopic and non-amblyopic eyes between all three amblyopia groups.
Keywords: Anisometropic amblyopia, optical coherence tomography, Retinal nerve fiber thickness (RNFLT), strabismic amblyopia
Amblyopia is defined as a unilateral or bilateral decrease of best-corrected visual acuity (BCVA) not attributable to structural or pathological ocular anomalies of the eyes and visual pathways.[1] It occurs in 2% to 4% of the general population.[1] Prior histological studies have established structural changes in the lateral geniculate body (LGB) and visual cortex in monkeys’ stimulus deprivation, anisometropic, and strabismic amblyopia.[2,3,4,5,6,7,8,9,10,11,12] However, the effect of an amblyopic stimulus on the retinal ganglion cells is relatively less well-reported, and limited studies suggest variable results. Various authors found no difference in the thickness of the retinal nerve fiber layer (RNFL) between amblyopic and healthy eyes.[13,14,15] In contrast, In contrast, Yen et al. and Yoon et al. reported a significant difference in RNFL thickness in eyes with anisometropic amblyopia compared with normal eyes.[16,17]
With the introduction of imaging modalities like the scanning laser polarimetry and Optical coherence Tomography (OCT), it has become possible to objectively quantify the peripapillary RNFLT (retinal nerve fiber layer thickness) and macular thickness. The Stratus OCT-3 (Carl Zeiss Meditec, Dublin, USA) provides in vitro, high-resolution images of RNFL equivalent to 10 mm histological sections of the retina.[18] GDx VCC (software version 5.5; Carl Zeiss, San Diego, USA) is an improved modified version of scanning laser polarimeter with variable corneal compensation that compensates for the corneal birefringence.[19]
A few studies have measured RNFLT in amblyopic patients with Scanning laser polarimetry[13,14] or OCT.[15,16] However, there is no published report where RNFLT has been measured in the same amblyopic population using both technologies to the best of our knowledge. Further, in the current study, we tested the hypothesis that eyes with anisometropic amblyopia have an increased RNFLT and macular thickness compared with the normal eye using the scanning laser polarimetry (GDx) and Stratus OCT.
Methods
The study was conducted on consecutive patients diagnosed with amblyopia seen at our institute from July 2004 to February 2006. The institutional ethics committee approved the study protocol and the methods adhered to the tenets of the declaration of Helsinki for the use of human subjects in biomedical research. Inclusion criteria were: BCVA ≥20/20 in the better eye, age between 5 years and 35 years, intraocular pressure (IOP) <22 mmHg in both eyes, clear ocular media, normal fundus examination, and unilateral amblyopia due to strabismus, anisometropia, or both.
We excluded subjects with recent intraocular surgery within 6 months, bilateral (emmetropic) amblyopia, deprivation amblyopia, coexisting nystagmus, and any other coexisting macular or retinal pathology that could affect final BCVA. We also excluded patients with pathologies that could affect the RNFL measurement (like cataract, retinal/macular pathology, glaucoma, abnormal discs/tilted discs, presence of systemic diseases, or neurological disorders producing RNFL damage), and in whom OCT images had a quality score <8 (or signal to noise ratio, SNR <33) or GDx VCC images with score <8, and GDx VCC images with atypical birefringence pattern (ABP). We also excluded patients unwilling to participate in the study.
Amblyopia was defined as reduction in the BCVA in the affected eye atleast two lines attributable to anisometropia, strabismus, or both, in the absence of abnormality in clinical examination.
Anisometropic amblyopia was defined as decreased BCVA secondary to uncorrected refractive error without strabismus with a difference in refractive error of ≥ 2.0 DS (Diopter sphere) or ≥ 1.50 D difference in astigmatism between corresponding meridians in the two eyes.
Strabismic amblyopia was defined as decreased BCVA secondary to manifest horizontal or vertical strabismus with a difference in the refractive error of ≤1.0 DS or spherical equivalent between the two eyes.
Mixed amblyopia was defined as decreased BCVA secondary to uncorrected refractive error and strabismus with a difference of >2.0 DS or spherical equivalent between the two eyes.
Anisometropia without amblyopia was defined as a difference in refractive error of >2.0 DS or spherical equivalent between the two eyes. However, the difference of BCVA was <2 lines between the two eyes.
We included patients with anisometropic amblyopia, strabismic amblyopia, mixed amblyopia, and anisometropia without amblyopia. All patients underwent a complete ophthalmic examination, including BCVA testing (Log MAR chart), cover test, ocular motility evaluation, measurement of ocular deviation using the prism bar, slit lamp examination, applanation tonometry, optic disc, and RNFL examination with a 60D/78D/90D lens, fundus examination with indirect ophthalmoscopy, axial length measurement with A-scan, keratometry, peripapillary RNFL measurement with GDx VCC and OCT Stratus 3.
Peripapillary RNFL thickness measurement
Peripapillary RNFL and macular RNFL were measured with OCT Stratus 3, version 4 (which uses the principles of low-coherence interferometry.[18] The fast RNFL algorithm was used to obtain RNFL thickness measurements. Three images were acquired from each subject. Each image consisted of 256 A-scans along a 3.4-mm diameter circular ring around the optic disc. OCT images were accepted if they were focused, optic nerve head was centered, and image quality score was ≥8 in both eyes. Peripapillary RNFL thickness parameters were automatically calculated by existing Stratus OCT software. The following parameters were evaluated in this study: average thickness (360° measurement), temporal quadrant thickness (316° to 45°), superior quadrant thickness (46° to 135°), nasal quadrant thickness (136° to 225°), inferior quadrant thickness (226° to 315°), and thickness for each of 12 clock-hour positions.
GDx VCC (Scanning Laser Polarimetry)
All patients underwent imaging using a commercially available scanning laser polarimeter (GDx VCC; software version 5.5; Carl Zeiss, San Diego, USA). The machine’s principle has been described in the literature.[19] A baseline image was automatically created from three images obtained for each subject. Images were accepted if they were focused, had the optic nerve head centered, and had an image score of ≥8. Based on the retardation map pattern, images were classified as normal birefringence pattern (NBP) and ABP. NBP images were defined as retardation maps with the highest retardation superiorly and inferiorly and low retardation nasally and temporally. ABP images were defined as retardation maps with alternating peripapillary circumferential bands of low and high retardation and variable areas of high retardation arranged in a spoke-like peripapillary pattern or splotchy areas of high retardation nasally and temporally.[19]
The GDx VCC software calculates summary parameters based on quadrants that are defined as temporal (335° to 24°), superior (25° to 144°), nasal (145° to 214°), or inferior (215° to 334°). The following GDx VCC parameters were investigated: TSNIT average, TSNIT standard deviation, superior average, inferior average, temporal average, and nasal average.
A sample size of at least 20 subjects in each group was required to detect a difference of 10 m in average RNFLT between amblyopia and controls. For analysis, the normal eye of the amblyopic patient served as a control eye. The paired t-test was used to compare peripapillary RNFL thickness between amblyopic and normal eyes in each group. RNFLT in the three amblyopic groups was compared using the analysis of variance test. Tests were considered statistically significant at a cut-off value of P < 0.05. Bonferroni’s method was used for adjusting the P value while performing multiple statistical comparisons.[20]
Macular thickness measurements
Fast-Macular Thickness protocol of Stratus OCT was used to obtain macular thickness measurements. The Stratus OCT software automatically calculated macular thickness parameters (version 4). Parameters compared in this study were foveal thickness, superior outer macular thickness, inferior outer macular thickness, temporal outer macular thickness, nasal outer macular thickness, superior inner macular thickness, inferior inner macular thickness, temporal inner macular thickness, nasal inner macular thickness, and average macular thickness.
Results
Thirty-seven eyes (37 patients) with anisometropic amblyopia, 22 eyes (22 patients) with strabismic amblyopia, 40 eyes (40 patients) with mixed amblyopia, and 9 eyes (9 patients) with anisometropia without amblyopia were included. Thirty-three eyes (33 patients) with anisometropic amblyopia, 20 eyes (20 patients) with strabismic amblyopia, 38 eyes (38 patients) with mixed amblyopia, and 9 eyes (9 patients) with anisometropia without amblyopia fulfilled study criteria and were included for the study. Fig. 1 shows the flowchart of inclusion of study patients. Table 1 shows the demographic features of patients in each group.
Figure 1.
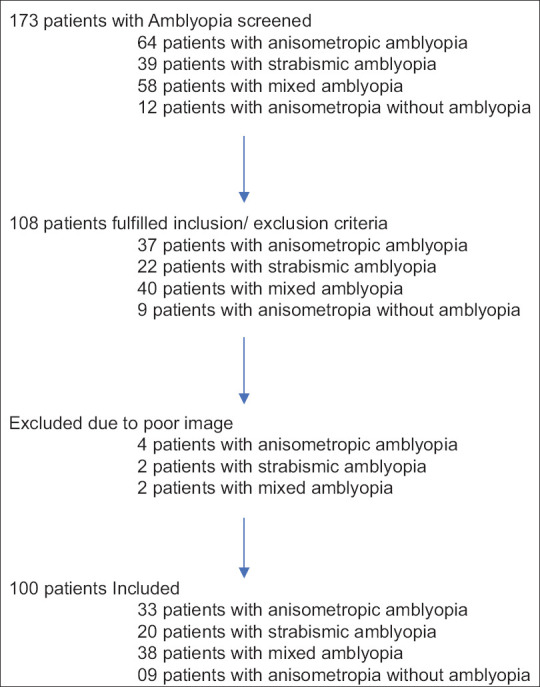
Flowchart of inclusion of study patients
Table 1.
Demographic features of patients in all study groups
| Anisometropic amblyopia Mean (SD), n=33 | Strabismic amblyopia (SD), n=20 | Mixed amblyopia (SD), n=38 | Anisometropia without amblyopia (SD), n=09 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| HA | N | MA | N | SA | N | MA | N | AA | N | |
| Numbers | 24 | 24 | 09 | 09 | 20 | 20 | 38 | 38 | 09 | 09 |
| Age (years) | 16.1 (7.2) | 15.8 (6.9) | 14.8 (6.4) | 16.8 (7.1) | 14.9 (7.4) | |||||
| Male: Female | 19: 5 | 05: 4 | 14:6 | 31:07 | 2:7 | |||||
| Refractive error (diopter, D) | 4.75 (2.75) | 1.75 (1.25) | −4.25 (3.5) | −1.37 (1.5) | 1.77 (3.60) | 1.67 (3.56) | 1.04 (5.54) | 0.74 (3.32) | −3.88 (2.90) | −1.94 (2.61) |
| Axial Length (mm) | 22.30 (1.05) | 22.60 (1.01) | 24.99 (1.97) | 23.87 (2.25) | 23.12 (0.94) | 23.05 (1.32) | 23.83 (2.11) | 23.33 (1.76) | 24.03 (2.05) | 23.28 (1.96) |
HA: Hypermetropia with amblyopia. MA: Myopic Amblyopia. SA: Strabismic Amblyopia. AA: Anisometropia without amblyopia. N: contralateral Normal Eye. SD: Standard Deviation. mm: millimeter
Table 2 shows the comparison of peripapillary parameters on measurement with OCT-3 in the different amblyopia groups. Average RNFL thickness in anisometropic amblyopia (98.21 m) and strabismic amblyopia (93.11 m) was similar, and the difference was statistically insignificant compared with the fellow normal eyes (P = 0.5 and 0.6, respectively). All RNFL parameters in amblyopia groups were not statistically significantly different from the normal group.
Table 2.
Comparison of retinal nerve fiber layer thickness using Optical coherence tomography (TD-OCT, OCT 3) parameters in different groups as compared with the normal fellow eye
| Parameter | Anisometropic amblyopia (SD) | Normal (SD) | P | Mixed amblyopia (SD) | normal (SD) | P | Strabismic amblyopia (SD) | normal (SD) | P | Anisometropia without amblyopia (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Superior Avg | 124.34 (21.36) | 128.75 (17.69) | 0.24 | 118.18 (26.88) | 126 (18.06) | 0.08 | 110.44 (23.28) | 119.98 (25.38) | 0.1 | 122.81 (22.01) |
| Inferior Avg | 116.02 (24.02) | 122.68 (22.72) | 0.13 | 116.22 (24.22) | 114.81 (20.37) | 0.67 | 118.35 (29.19) | 113.24 (21.3) | 0.3 | 111.26 (20.03) |
| Temporal Avg | 72.39 (27.36) | 62.59 (12.84) | 0.05 | 74.94 (25.4) | 67.04 (13.79) | 0.03 | 63.35 (16.23) | 70.83 (18.8) | 0.16 | 62.56 (15.21) |
| Nasal Avg | 80.07 (31.62) | 85.37 (21.10) | 0.36 | 80.67 (25.75) | 80.53 (23.79) | 0.97 | 80.33 (26.53) | 77.10 (23.43) | 0.62 | 78.70 (25.49) |
| Avg Thickness | 98.21 (16.27) | 99.84 (12.8) | 0.5 | 97.48 (15.12) | 93.11 (19.91) | 0.84 | 93.11 (14.95) | 95.26 (17.56) | 0.62 | (14.55) |
SD: Standard Deviation. Avg: Average. Statistical significance at 0.005 using Bonferroni correction
Table 3 shows the comparison of peripapillary parameters with GDx VCC in the different amblyopia groups. The difference between all the peripapillary parameters in strabismic amblyopia, anisometropic amblyopia, and mixed amblyopia compared with the normal group was not statistically significant.
Table 3.
Comparison of GDx VCC parameters in different study groups as compared with the normal fellow eye
| Parameter | Anisometropic amblyopia µ (SD) | Normal µ (SD) | P | Strabismic amblyopia µ (SD) | Normal µ (SD) | P | Mixed Amblyopia µ (SD) | Normal µ (SD) | P | Anisometropia without amblyopia µ (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| NFI | 21.24 (13.65) | 17.35 (8.82) | 0.11 | 25.7 (15.35) | 24.55 (14.94) | 0.7 | 22.36 (16.43) | 21.26 (14.12) | 0.73 | 24.71 (9.03) |
| TSNIT Avg | 61.2 (26.16) | 55.84 (5.39) | 0.25 | 53.38 (7.13) | 52.81 (7.9) | 0.73 | 59.27 (15.93) | 54.22 (9.13) | 0.1 | 52.07 (4.28) |
| Sup Avg | 69.47 (24.64) | 68.75 (8.69) | 0.87 | 62.62 (9.94) | 61.39 (10.17) | 0.55 | 68.19 (17.86) | 65.4 (11.06) | 0.38 | 60.96 (6.61) |
| Inf Avg | 68.71 (24.83) | 66.25 (9.65) | 0.57 | 61.3 (7.81) | 63.12 (8.61) | 0.36 | 64.23 (15.14) | 61.78 (9.43) | 0.43 | 58.62 (6.64) |
| TSNIT Std | 21.82 (7.35) | 25.39 (5.8) | 0.012 | 22.86 (4.44) | 24.39 (3.77) | 0.13 | 22.92 (5.99) | 23.33 (4.57) | 0.73 | 19.74 (5.42) |
SD: Standard Deviation. Avg: Average. NFI: Nerve Fiber Index. Statistical significance at 0.005 using Bonferroni correction
Table 4 shows the comparison of macular RNFL parameters measured with stratus OCT-3 in the different amblyopia groups. The total foveal thickness in the anisometropic amblyopia group was 152.82 (standard deviation, SD: 26.78) mm and 150.42 (SD: 23.84mm) in the fellow eye (P = 0.38). In the mixed amblyopia group, the foveal thickness was higher in the amblyopic eye compared with the normal eye (166.1 ± 36.85 mm vs 155.47 ± 29.94 mm) but did not reach statistical significance (P = 0.1, after Bonferroni correction). Differences between all the macular parameters with OCT in strabismic amblyopia, anisometropic amblyopia, and mixed amblyopia compared with the normal group were not statistically significant.
Table 4.
Comparison of Macular parameters with Stratus 3OCT in different Amblyopia groups to Normal
| Parameter | Anisometropic amblyopia µ (SD) | Normal µ (SD) | P | Strabismic amblyopia µ (SD) | Normal µ (SD) | P | mixed amblyopia µ (SD) | Normal µ (SD) | P | Anisometropia without amblyopia µ (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Foveal Thickness | 152.82 (26.78) | 150.42 (23.84) | 0.38 | 155.86 (32.99) | 153.27 (27.65) | 0.78 | 166.1 (36.85) | 155.47 (29.94) | 0.04 | 140.33 (22.26) |
| Total Macular vol | 6.31 (0.55) | 6.42 (0.38) | 0.27 | 6.47 (0.49) | 6.33 (0.5) | 0.14 | 6.38 (0.43) | 6.35 (0.42) | 0.55 | 6.05 (0.42) |
| Outer Sup Avg Vol | 1.18 (0.12) | 1.23 (0.1) | 0.07 | 1.21 (0.08) | 1.19 (0.08) | 0.05 | 1.19 (0.08) | 1.19 (0.08) | 0.9 | 1.14 (0.09 |
| Outer Inf Avg Vol | 1.11 (0.13) | 1.12 (0.07) | 0.46 | 1.15 (0.1) | 1.12 (0.11) | 0.09 | 1.13 (0.09) | 1.12 (0.09) | 0.58 | 1.08 (0.08) |
| Outer Temporal Avg vol | 1.1 (0.08) | 1.1 (0.06) | 0.54 | 1.12 (0.09) | 1.09 (0.1) | 0.11 | 1.09 (0.08) | 1.09 (0.09) | 0.56 | 1.02 (0.1) |
| Outer Nasal Avg Vol | 1.27 (0.12) | 1.28 (0.08) | 0.54 | 1.29 (0.14) | 1.28 (0.13) | 0.65 | 1.28 (0.11) | 1.27 (0.09) | 0.34 | 1.21 (0.08) |
| Inner Sup Avg Vol | 0.38 (0.04) | 0.39 (0.03) | 0.2 | 0.39 (0.03) | 0.38 (0.03) | 0.55 | 0.38 (0.03) | 0.38 (0.03) | 0.82 | 0.37 (0.02) |
| Inner Inf Avg Vol | 0.38 (0.04) | 0.39 (0.03) | 0.24 | 0.4 (0.03) | 0.39 (0.03) | 0.13 | 0.40 (0.03) | 0.39 (0.03) | 0.83 | 0.37 (0.03) |
| Inner Temporal Avg vol | 0.36 (0.04) | 0.37 (0.03) | 0.2 | 0.37 (0.03) | 0.37 (0.03) | 0.87 | 0.37 (0.03) | 0.37 (0.03) | 0.55 | 0.35 (0.02) |
| Inner Nasal Avg Vol | 0.38 (0.05) | 0.39 (0.03) | 0.43 | 0.39 (0.04) | 0.39 (0.03) | 0.61 | 0.38 (0.03) | 0.39 (0.03) | 0.9 | 0.37 (0.02) |
| Central Foveal Vol | 0.14 (0.02) | 0.14 (0.02) | 1 | 0.14 (0.02) | 0.14 (0.03) | 0.68 | 0.15 (0.02) | 0.14 (0.02) | 0.08 | 0.13 (0.02) |
SD: Standard Deviation. Avg: Average. Vol: volume. Statistical significance at 0.005 using Bonferroni correction
Discussion
Since Hubel and Wiesel’s pioneering work, studies have shown definite changes in the visual cortex and LGB areas that receive inputs from the amblyopic eye[4,5,6,7]; however, changes in the retinal nerve fiber layer have always remained speculative. It is proposed that there is a further arrest of apoptosis in amblyopic eyes. Hence, retinal nerve fiber layer thickness is likely to be higher. However, other hypotheses suggest possible degenerative changes in these patients with amblyopia. A histological study in monkeys could not demonstrate any retinal histological alterations even in the presence of pronounced arrest in LGB cell growth; it was proposed that parafoveal ganglion cell alterations occurring after a long period of visual deprivation are due to retrograde degeneration.[4] If the theory of retrograde degeneration in amblyopia is correct, RNFLT should be thinner; if amblyopia affects the postnatal reduction of ganglion cells, RNFL thickness may be thicker than in the normal eye.[5]
Using scanning laser polarimetry (GDx), Colen et al. reported slightly higher but statistically nonsignificant differences in RNFL thickness in normal eyes compared with 20 strabismic amblyopic eyes.[15] Using scanning laser polarimetry (GDx) in a small unilateral strabismic amblyopic group, Baddini-Caramelli et al. also reported no statistical difference in thickness of the nerve fiber layer in amblyopic and normal eyes.[14] Bozkurt et al.[13] reported no difference in RNFL thickness between the two eyes in patients with anisometropic and strabismic amblyopia using scanning laser polarimetry (GDx). Miki et al. also concluded that there is no significant change in the RNFLT in amblyopic eyes. They proposed that visual impairment in amblyopia is functional, and no organic changes can be attributed to these patients. They further reported that the RNFLT of amblyopic eyes is comparable to normal eyes. Our results are concordant with these published results. Sahin et al.[17] compared RNFL thickness between anisometropic patients (divided into three groups as hyperopic, myopic, and meridional/astigmatism) and normal fellow eyes and a control group. There was no statistically significant difference in RNFLT of superior and inferior quadrants. They concluded that the presence of amblyopia seems not to be related to RNFL-T. We have also obtained similar results in our study.
Yen et al.[15] measured RNFLT OCT with scan pattern “Nerve Head 2.0R” (Carl Zeiss Meditec, Dublin, CA) in 20 patients with strabismic amblyopia and 18 with anisometropic amblyopia. They reported higher RNFLT than the normal fellow eye and the strabismus amblyopia group. In contrast with their study, we observed that difference in average RNFLT was not statistically different from normal eyes, even though RNFL thickness was slightly thicker than in the strabismic amblyopia group. This difference could be due to differences in methodology between our group and in Yen et al.’s group. At least 7 of 20 subjects with strabismic amblyopia group also had anisometropia in Yen et al.’s group. We had a rigorous definition of the three groups in our group, and subjects with both anisometropia and strabismus were labeled as mixed amblyopia. It is possible that patient selection with both strabismus and anisometropia could have affected the results in the study by Yen et al.
Yoon et al.[16] measured both peripapillary and macular RNFL thickness with OCT in 31 patients with aniso-hypermetropic amblyopia. They reported thicker peripapillary RNFL in anisometropic amblyopic eyes compared with normal eyes. High hypermetropia may have thicker RNFL due to the small size of the eye, and the apparent higher thickness of RNFL may be due to this anatomical change in the eye rather than the effect of amblyopia. Unfortunately, we had included both hypermetropic and myopic anisometropia and could not obtain such results.
Andalib et al.[21] compared the macular and retinal nerve fiber layer thicknesses measured by OCT in amblyopic and fellow eyes. In the anisometropic group, mean macular thickness was significantly increased in the amblyopic eyes compared with fellow eyes, but peripapillary RNFLT values were similar. There was no significant difference in the strabismic group. They concluded that a thicker macula was found in eyes with anisometropic amblyopia. However, when we reanalyzed the data using Bonferroni correction for multiple comparisons, the difference was not statistically significant (P = 0.2). We obtained non-significant differences between the amblyopic and the normal fellow eye in anisometropic amblyopia.
Singh et al.[22] studied the difference in central macular thickness (CMT) and peripapillary retinal nerve fiber layer (RNFL) thickness in patients with anisometropic amblyopia using SD-OCT. There was no significant difference in the CMT of the better and worse eyes in anisomyopia, anisohypermetropia, or anisoastigmatism. They concluded that there was no significant difference in CMT and peripapillary RNFL thickness in anisomyopia and anisoastigmatism. However, they observed that the inferior quadrant RNFL was significantly thicker as in patients with anisohypermetropia compared with the fellow eye. However, if we adjust for multiple comparisons in their study using Bonferroni correction, this difference was not statistically significant (P = 0.06).
Kasem et al.[23] investigated the changes in macular parameters (thickness, volume) and peripapillary retinal nerve fiber layer (RNFL) thickness (RNFLT) in different cases of amblyopia versus the healthy fellow eyes using OCT. There were significant differences in mean CMT, mean average macular thickness, mean macular volume, and the mean global RNFLT in the amblyopic eyes versus the fellow eyes. Age and axial length were the only independent variables that statistically significantly correlated with the CMT. They concluded that unilateral amblyopic eyes were prone to have a higher CMT and thicker global RNFL than those of the healthy fellow eyes. However, we could not find any significant difference in the amblyopia group.
Using SD-OCT, Chen et al.[24] compared the macular and RNFL thickness in children with anisometropic amblyopia. They reported that the average thickness of the outer macular ring and RNFL were significantly thicker in eyes with anisometropic amblyopia than those with emmetropia. However, following adjustment for axial length and refractive error, this difference was not significant. Furthermore, the macular parameters were not different between treated and untreated amblyopic eyes in their group. They concluded that macular and RNFL thicknesses appear to be more extensively associated with differences in axial length and refraction than with amblyopic development.
Araki et al.[25] investigated macular retinal and choroidal thickness in eyes with anisometropia and strabismus compared with that in fellow and normal eyes using swept-source OCT (SS-OCT). In both amblyopia groups (anisohypermetropic amblyopia and strabismic amblyopia without anisometropia), there were no significant differences in the mRNFL, GCL + IPL, and GCC thicknesses among the amblyopic, fellow, and control eyes. In the anisometropic amblyopia group, choroidal thicknesses of amblyopic eyes were significantly higher than that of fellow and normal eyes. In contrast, the choroidal thicknesses were not significantly different in the strabismic amblyopia group. They concluded that the discrepancy in choroidal thickness between the two types of amblyopia might be due to differences in ocular size and the underlying mechanism. We did not find any difference in RNFLT; however, we did not measure the choroidal thickness. Various other authors have also reported no difference in macular or peripapillary RNFL thickness in amblyopic eyes than contralateral normal eyes.[26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]
Recently, Kavitha et al.[41] investigated the effects of occlusion therapy in unilateral anisometropic amblyopia on macular, foveal, and retinal nerve fiber layer thickness using OCT. The study showed a reduction in the average macular and foveal thickness of amblyopic eyes following compliant amblyopia therapy but no significant change in the age-matched controls. In addition, there was no statistically significant change in the overall RNFL thickness. As we have not collected post-occlusion therapy data, we cannot comment on such changes in our group. However, we did not find any baseline difference between macular parameters in any amblyopia group.
Table 5 compares published literature for various amblyopia groups using different imaging technologies. It shows that there is no consensus among various studies. For example, some studies show thick RNFLT, while some show thick macular parameters in anisometropic eyes compared with fellow eyes. At the same time, significant published reports failed to show the difference (either RNFL or macular parameters) in amblyopic eyes.
Table 5.
Compares various published reports of RNFL and macular parameters in amblyopia groups
| Author | Types of patients (with sample size) | Machine | RNFL parameters | Macular parameters | Specific comments |
|---|---|---|---|---|---|
| Colen et al.[14] | strabismic amblyopia (20 eyes) | GDx | Statistically non-significant | Not measured | |
| Baddini-Caramelli et al.[12] | strabismic amblyopia (21 eyes) | GDx | Statistically non-significant | Not measured | |
| Bozkurt et al.[13] | Anisometropic (18), two strabismic (2), and mixed (4) amblyopic eyes | GDx | Statistically non-significant | Not measured | |
| Miki et al.[26] | Persistent unilateral amblyopia (26 patients) and recovered unilateral amblyopia (25 patients) | Stratus OCT | Statistically non-significant | Not measured | No significant difference in the RNFLT between the persistently amblyopic eyes and the previously amblyopic eyes |
| Sahin G[17] | Anisometropic amblyopia (74 eyes) | Stratus OCT | Statistically non-significant | Not measured | |
| Yen et al.[15] | Strabismic amblyopia (20 eyes) & Anisometropic amblyopia (19 eyes) | Stratus OCT | Thicker RNFLT in Anisometropic amblyopia | Not measured | 7 of 20 subjects with strabismic amblyopia group had anisometropia |
| Yoon et al.[16] | aniso-hypermetropic amblyopia (31 eyes) | Stratus OCT | Thicker RNFLT | Statistically non-significant | Thicker RNFL due to the small size of the eye, and the apparent higher thickness of RNFL may be due to this anatomical change |
| Andalib et al.[21] | Anisometropic amblyopia (25 eyes) andstrabismic amblyopia (25 eyes) | OCT | No difference | Thick macular parameters in anisometropic amblyopia eyes | Adjusting for the multiple parameters, using Bonferroni correction, no statistical significance |
| Singh et al.[22] | myopic anisometropia (31 eyes), astigmatic anisometropia (28 eyes), hypermetropic anisometropia (42 eyes) | SD-OCT | No difference | No difference | |
| Kasem et al.[23] | Strabismic (22 eyes), anisometropic (30 eyes), deprivational amblyopia (12 eyes) | OCT | Thicker global RNFL | Higher CMT | |
| Chen et al.[24] | Anisometropic amblyopia (53 eyes), and fully corrected previous amblyopia (26 eyes) | SD-OCT | Thick RNFL in eyes with anisometropic amblyopia | Thick average thickness of outer macular ring in amblyopic eye | Adjusting for axial length and refractive error, no statistically significant difference |
| Araki et al.[25] | Strabismic (15 eyes), anisometropic amblyopia (31 eyes) | SD-OCT | No difference | No difference | |
| Kavitha et al.[41] | Anisometropic amblyopia (30 eyes) | SD-OCT | No difference | Thicker CMT, decreased in follow up after amblyopia therapy | There was no difference in RNFLT between amblyopic eyes and normal fellow eyes before and after occlusion therapy |
| Atakan et al.[44] | Strabismic (30 eyes), anisometropic amblyopia (31 eyes) | SD-OCT | No difference | No difference | TMT in strabismic group was thinner compared to anisometropic group but was not different compared to fellow normal eyes |
| Rajavi Z[45] | anisometropic amblyopia (44 eyes) | SD-OCT | No difference | No difference | Statistically significant thicker CMT in moderate to severe amblyopia |
| AL-Haddad et al.[46] | Anisometropic (31 eyes), strabismic (14 eyes) and mixed amblyopia, 20 eyes had mixed amblyopia | SD-OCT | No difference | No difference | |
| Alotaibi et al.[47] | Anisometropic (39 eyes), amblyopia | OCT | Thick RNFLT in all amblyopia group | No Difference | |
| Present study | Anisometropic amblyopia (33 eyes), strabismic amblyopia (20 eyes), mixed amblyopia (38 eyes) | Stratus OCT, GDx | No difference | No difference | RNFLT measured by two different devices |
Our study has a few limitations. RNFL data was collected on Stratus OCT (time-based) instead of spectral-domain OCT. However, various publications have compared Time-domain OCT versus Spectral-domain OCT, which suggests differences in RNFL thickness between two machines but the excellent correlation in all parameters.[42,43]
Conclusion
As reported in prior literature, we did not observe differences in peripapillary RNFLT in anisometropic amblyopia than normal eyes. Therefore, our finding may suggest that no structural abnormalities are detected in RNFL and macular thickness in amblyopic eyes, and the cause for visual impairment in these patients is likely functional.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Day S. Normal and abnormal visual development. In: Taylor D, editor. Pediatric Ophthalmology. London: Blackwell Scientific Publications; 1990. pp. 7–20. [Google Scholar]
- 2.Headon MP, Powell TP. Celluar change in the lateral geniculate nucleus of infant monkeys after suture of the eyelids. J Anat. 1973;116:135–45. [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman SM, Wilson JR. behavioral and morphological evidence for binocular competition in the postnatal development of the dog's visual system. J Comp Neurol. 1975;161:183–95. doi: 10.1002/cne.901610204. [DOI] [PubMed] [Google Scholar]
- 4.Von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol Vis Sci. 1973;12:727–38. [PubMed] [Google Scholar]
- 5.Wiesel T, Hubel DH. Effects of visual deprivation on morphology and physiology of cell in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–93. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 6.Von Noorden GK, Crawford MLJ, Levancy RA. The lateral geniculate nucleus in human anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1983;24:788–90. [PubMed] [Google Scholar]
- 7.Von Noorden GK, Crawford MLJ. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992;33:2729–32. [PubMed] [Google Scholar]
- 8.Wiesel TN, Hubel DH. Single-cell response in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 9.Baker FH, Von Noorden GK. Effects of visual deprivation and strabismus on the response of neurons in the visual cortex of the monkey, including studies on the striate and pre striate cortex in the normal animal. Brain Res. 1974;66:185–208. [Google Scholar]
- 10.Crawford MLJ, Blake R, Cool SJ, Von Noorden GK. Physiological consequences of unilateral and bilateral eye closure in macaque monkeys:Some further observations. Brain Res. 1975;85:150–4. doi: 10.1016/0006-8993(75)90809-4. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MLJ, von Noorden GK. Optical induced concomitant strabismus in monkeys. Invest Ophthalmol Vis Sci. 1980;19:1105–9. [PubMed] [Google Scholar]
- 12.Baddini-Caramelli C, Hatanaka M, Polati M, Umino AT, Susanna R., Jr Thickness of the retinal nerve fiber layer in amblyopic and normal eyes:A scanning laser polarimetry study. J AAPOS. 2001;5:82–4. doi: 10.1067/mpa.2001.112678. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt B, Irkec M, Orhan M, Karaagaoglu E. Thickness of the retinal nerve fiber layer in patients with anisometropic and strabismic amblyopia. Strabismus. 2003;11:1–7. doi: 10.1076/stra.11.1.1.14091. [DOI] [PubMed] [Google Scholar]
- 14.Colen TP, de Faber JT, Lemij HG. The Rotterdam Eye Hospital, Rotterdam, The Netherlands. Retinal nerve fiber layer thickness in human strabismic amblyopia Binocul Vis Strabismus Q. 2000;15:141–6. [PubMed] [Google Scholar]
- 15.Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004;45:2224–30. doi: 10.1167/iovs.03-0297. [DOI] [PubMed] [Google Scholar]
- 16.Yoon SW, Park WH, Baek SH, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005;19:62–7. doi: 10.3341/kjo.2005.19.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Sahin G, Dal D. Analysis of retinal nerve fiber layer thickness in anisometropic amblyopia via optic coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2019;257:2103–10. doi: 10.1007/s00417-019-04402-2. [DOI] [PubMed] [Google Scholar]
- 18.Swanson EA, Izatt JA, Hee MR, Huang D, Lin CP, Schuman JS, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18:1864–6. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Weinreb RN. Individualized compensation of anterior segment birefringence during scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2002;43:2221–8. [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Multiple significance tests:The Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andalib D, Javadzadeh A, Nabai R, Amizadeh Y. Macular and retinal nerve fiber layer thickness in unilateral anisometropic or strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2013;50:218–21. doi: 10.3928/01913913-20130319-02. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Rohatgi J, Gupta VP, Kumar V. Measurement of peripapillary retinal nerve fiber layer thickness and macular thickness in anisometropia using spectral-domain optical coherence tomography:A prospective study. Clin Ophthalmol. 2017;2311:429–34. doi: 10.2147/OPTH.S123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasem MA, Badawi AE. Changes in macular parameters in different types of amblyopia:Optical coherence tomography study. Clin Ophthalmol. 2017;11:1407–16. doi: 10.2147/OPTH.S143223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Chen J, Huang J, Xu J, Zhang F, Lu F. Comparison of macular and retinal nerve fiber layer thickness in untreated and treated binocular amblyopia. Curr Eye Res. 2013;38:1248–54. doi: 10.3109/02713683.2013.805233. [DOI] [PubMed] [Google Scholar]
- 25.Araki S, Miki A, Goto K, Yamashita T, Takizawa G, Haruishi K, et al. Macular retinal and choroidal thickness in unilateral amblyopia using swept-source optical coherence tomography. BMC Ophthalmol. 2017;17:167. doi: 10.1186/s12886-017-0559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki A, Shirakashi M, Yaoeda K, Kabasawa Y, Ueki S, Takagi M, et al. Retinal nerve fiber layer thickness in recovered and persistent amblyopia. Clin Ophthalmol. 2010;20(4):1061–4. doi: 10.2147/opth.s13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Lu F, Liu W, Zhang F, Chen W, Chen J. Retinal nerve fibre layer thickness and macular thickness in patients with esotropic amblyopia. Clin Exp Optom. 2013;96:267–71. doi: 10.1111/cxo.12001. [DOI] [PubMed] [Google Scholar]
- 28.Kee SY, Lee SY, Lee YC. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006;20:177–81. doi: 10.3341/kjo.2006.20.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lekskul A, Wuthisiri W, Padungkiatsagul T. Evaluation of retinal structure in unilateral amblyopia using spectral-domain optical coherence tomography. J AAPOS. 2018;22:386–9. doi: 10.1016/j.jaapos.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006;142:247–51. doi: 10.1016/j.ajo.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Ersan I, Zengin N, Bozkurt B, Ozkagnici A. Evaluation of retinal nerve fiber layer thickness in patients with anisometropic and strabismic amblyopia using optical coherence tomography. J Pediatr Ophthalmol Strabismus. 2013;50:113–7. doi: 10.3928/01913913-20121211-02. [DOI] [PubMed] [Google Scholar]
- 32.Altintas O, Yüksel N, Ozkan B, Caglar Y. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005;42:216–21. doi: 10.3928/01913913-20050701-03. [DOI] [PubMed] [Google Scholar]
- 33.Walker RA, Rubab S, Voll AR, Erraguntla V, Murphy PH. Macular and peripapillary retinal nerve fibre layer thickness in adults with amblyopia. Can J Ophthalmol. 2011;46:425–7. doi: 10.1016/j.jcjo.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Yakar K, Kan E, Alan A, Alp MH, Ceylan T. Retinal nerve fibre layer and macular thicknesses in adults with hyperopic anisometropic amblyopia. J Ophthalmol. 2015;2015:946467. doi: 10.1155/2015/946467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altıntaş Ö, Gümüştaş S, Cinik R, Anık Y, Özkan B, Karabaş L. Correlation of the measurements of optical coherence tomography and diffuse tension imaging of optic pathways in amblyopia. Int Ophthalmol. 2017;37:85–93. doi: 10.1007/s10792-016-0229-0. [DOI] [PubMed] [Google Scholar]
- 36.Kusbeci T, Karti O, Karahan E, Oguztoreli M. The evaluation of anatomic and functional changes in unilateral moderate amblyopic eyes using optical coherence tomography and pupil cycle time. Curr Eye Res. 2017;42:1725–32. doi: 10.1080/02713683.2017.1349153. [DOI] [PubMed] [Google Scholar]
- 37.Tugcu B, Araz-Ersan B, Erdogan ET, Tarakcioglu H, Coskun C, Yigit U, et al. Structural and functional comparison of the persistent and resolved amblyopia. Doc Ophthalmol. 2014;128:101–9. doi: 10.1007/s10633-013-9422-x. [DOI] [PubMed] [Google Scholar]
- 38.Tugcu B, Araz-Ersan B, Kilic M, Erdogan ET, Yigit U, Karamursel S. The morpho-functional evaluation of retina in amblyopia. Curr Eye Res. 2013;38:802–9. doi: 10.3109/02713683.2013.779721. [DOI] [PubMed] [Google Scholar]
- 39.TaşkıranÇömez A, Şanal Ulu E, Ekim Y. Retina and optic disc characteristics in amblyopic and non-amblyopic eyes of patients with myopic or hyperopic anisometropia. Turk J Ophthalmol. 2017;47:28–33. doi: 10.4274/tjo.54289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang BZ, Taranath D. A comparison between the amblyopic eye and normal fellow eye ocular architecture in children with hyperopic anisometropic amblyopia. J AAPOS. 2012;16:428–30. doi: 10.1016/j.jaapos.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Kavitha V, Heralgi MM, Harishkumar PD, Harogoppa S, Shivaswamy HM, Geetha H. Analysis of macular, foveal, and retinal nerve fiber layer thickness in children with unilateral anisometropic amblyopia and their changes following occlusion therapy. Indian J Ophthalmol. 2019;67:1016–22. doi: 10.4103/ijo.IJO_1438_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Fan N, Shen X, He J. Comparison of the diagnostic ability of retinal nerve fiber layer thickness measured using time domain and spectral-domain optical coherence tomography in primary open-angle glaucoma. Eye Sci. 2011;26:132–7. doi: 10.3969/j.issn.1000-4432.2011.03.002. 142. [DOI] [PubMed] [Google Scholar]
- 43.Takagishi M, Hirooka K, Baba T, Mizote M, Shiraga F. Comparison of retinalnerve fiber layer thickness measurements using time domain and spectral-domain optical coherence tomography, and visual field sensitivity. J Glaucoma. 2011;20:383–7. doi: 10.1097/IJG.0b013e3181efb371. [DOI] [PubMed] [Google Scholar]
- 44.Atakan M, Culfa S, Calli U, Penbe AD, Atakan TG. Evaluation of retinal nerve fibre layer and macular thickness in amblyopia. J Clin Exp Ophthalmol. 2015;6:437. [Google Scholar]
- 45.Rajavi Z, Moghadasifar H, Feizi M, Haftabadi N, Hadavand MR, Yaseri M, et al. Macular thickness and amblyopia. J Ophthalmic Vis Res. 2014;9:478–83. doi: 10.4103/2008-322X.150827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Haddad CE, Mollayess GM, Cherfan CG, Jaafar DF, Bashshur ZF. Retinal nerve fibre layer and macular thickness in amblyopia as measured by spectral-domain optical coherence tomography. Br J Ophthalmol. 2011;95:1696–9. doi: 10.1136/bjo.2010.195081. [DOI] [PubMed] [Google Scholar]
- 47.Alotaibi AG, Al Enazi B. Unilateral amblyopia:Optical coherence tomography findings. Saudi J Ophthalmol. 2011;25:405–9. doi: 10.1016/j.sjopt.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


