Figure 12.
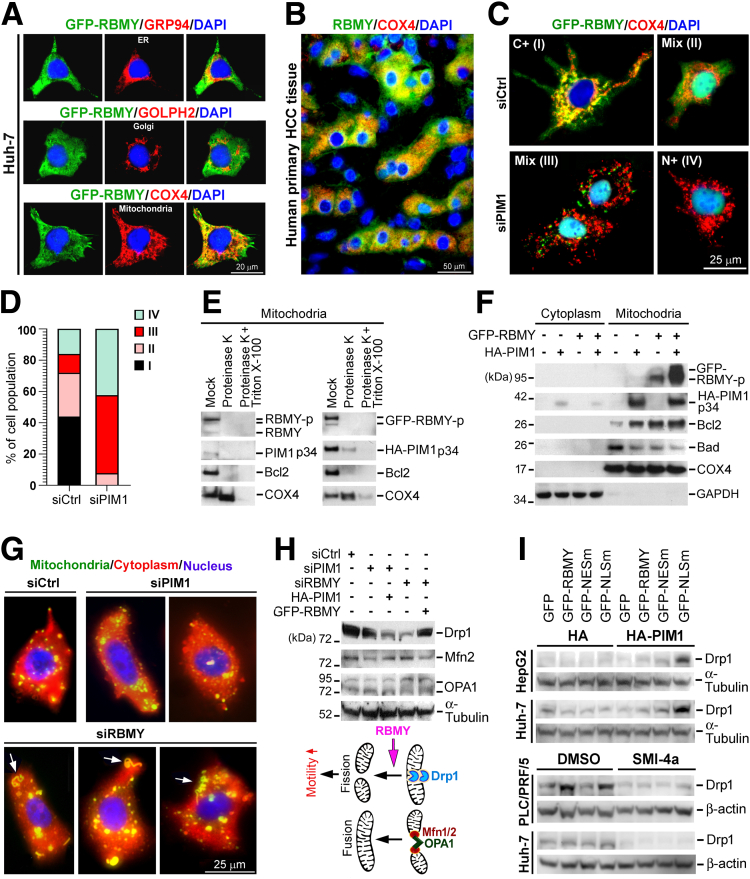
PIM1 fosters the mitochondrial translocation of RBMY to regulate mitochondrial integrity and dynamics. Subcytoplasmic localization of RBMY was analyzed by double-stained IFA probing for the colocalization of RBMY and organelle markers in (A) GFP-RBMY–transfected Huh-7 cells and (B) primary HCC tissue. Glucose-regulated protein of 94 kDa (GRP94), Golgi phosphoprotein 2 (GOLPH2), and COX4 are markers of endoplasmic reticulum (ER), Golgi, and mitochondria, respectively. (C and D) IFA showed the distribution of GFP-RBMY in Huh-7 cells transfected 3 times with siCtrl or siPIM1. (D) The percentage of the cell population (means ± SDs, Student t test) was determined with respect to the different subcellular distribution patterns of GFP-RBMY, which are indicated as patterns I–IV. (E) Immunoblotting showed the submitochondrial localization of PIM1 and RBMY. Mitochondria isolated from Huh-7 cells 24 hours post-transfection were subjected to proteinase K and/or Triton X-100 treatment and analyzed by Western blot. Mock indicates the basal levels of mitochondrial proteins. Bcl2 and COX4 represent OMM and IMM proteins, respectively. (F) Mitochondrial levels of apoptosis-related proteins (Bcl2 and Bad) upon overexpression of HA-PIM1 and GFP-RBMY. After subcellular fractionation, the isolated cytoplasmic and mitochondrial fractions were analyzed by Western blot. COX4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used to indicate the purity of the mitochondrial and cytoplasmic fractions, respectively. (G) Fluorescence staining of the mitochondria, cytoplasm, and nucleus of live PLC/PRF/5 cells with PIM1 or RBMY depletion using Mito-ID Green, CytoPainter Red, and Hoechst 33342, respectively. The white arrows indicate mitochondrial fusion. (H) Immunoblotting of PLC/PRF/5 cells transfected 3 times with the indicated siRNAs and once with HA-PIM1 or GFP-RBMY. A hypothetical model of the mechanism by which RBMY regulates mitochondrial dynamics is presented. (I) Western blot analyzed the Drp1 levels of the indicated cells expressing GFP-RBMY WT or mutants together with HA-PIM1 (upper) and kinase inhibitor SMI-4a (lower). Cells were treated with SMI-4a (30 μmol/L) or solvent control (dimethyl sulfoxide [DMSO]) 24 hours before transfecting with GFP-RBMY-WT or mutants. These cells were continuously supplied with SMI-4a and harvested after 24 hours post-transfection. DAPI, 4′,6-diamidino-2-phenylindole.