Abstract
Severe granulomatous chronic villitis with focal remnants of Toxoplasma was confirmed by immunohistochemistry and DNA-based methods in the placenta from a child that died four days after birth. The immunocompetent mother was seronegative for Toxoplasma at delivery and 10 months later. Placental infection may happen without maternal systemic infection.
Keywords: Toxoplasma gondii, Placenta, Immunology, Neonatal, Infant
1. Background
Toxoplasmosis is caused by Toxoplasma gondii and can be either acquired or congenital (Dunay et al., 2018). Congenital toxoplasmosis (CT) is considered to be due to vertical transmission of T. gondii infection from a systemically infected mother to the fetus. In a recent study from France, it was reported that about 31% of pregnant women had antibodies against T. gondii (age-adjusted data) (Robinson et al., 2021), suggesting that almost 1/3 of pregnant women have been exposed to the parasite at some point in their lives. Pregnant women who have not seroconverted are at particular risk of transmitting T. gondii to their fetus, if they become infected during pregnancy (Dunay et al., 2018). In immunocompetent women, infection with T. gondii has been thought always to lead to seroconversion.
In support of this assumption, we were unable to identify cases in the literature in which CT is observed or suspected in infants born to seronegative mothers. Meanwhile, a recent study identified a few seronegative women with T. gondii PCR-positive placentas (Matin et al., 2017). However, that study was limited by lack of serologic follow-up data and incomplete diagnostic workup. Moreover, a case of a seropositive child born to a seronegative woman was described by Armstrong et al. in 2004 (Armstrong et al., 2004); however, the serologic workup of the mother was limited in that study.
In this report, we describe a case of T. gondii-associated placentitis in the absence of maternal seroconversion with preterm delivery and death of the infant at four days of age.
2. Case report
A 29-year-old nulliparous woman in gestational week (GW) 25 + 6 and with no known conventional risk factors for developing toxoplasmosis (e.g., exposure to cats or ingestion of undercooked meat) presented to the delivery ward due to spontaneous contractions. She was fully dilated with exposed membranes. Apart from an episode of vaginal bleeding in GW 16, the pregnancy had been uncomplicated. The vaginal delivery was complicated due to face presentation and severe asphyxia with a venous cord blood pH of 6.9 (normal value >7.24) and a base deficit of 13 mmol/L (normal value <8 mmol/L). The birthweight was normal for the gestational age, 915 g. Unfortunately, the infant died four days later due to multi-organ failure.
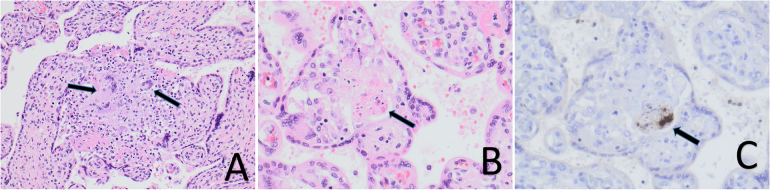
Pathoanatomical examination of the placenta revealed severe granulomatous chronic villitis with multinucleated giant cells (Fig. 1, A) and with focal remnants of Toxoplasma organisms (Fig. 1, B) reactive for Toxoplasma immunohistochemistry (Fig. 1, C). Toxoplasma gondii-specific DNA was detected in paraffin-embedded sections by multiple PCR methods (Table 1), including two widely used T. gondii-specific real-time PCR methods that target two different parts of the T. gondii genome, and a previously described metabarcoding assay (Stensvold et al., 2021). A small subunit ribosomal DNA consensus sequence was obtained from the DNA sequence output from the metabarcoding analysis (GenBank accession number, OM630157). This sequence was queried against the National Center for Biotechnology Information's Nucleotide Database and proved identical to T. gondii and few other Apicomplexan genera not described as potential human pathogens such as Hammondia and Neospora. A subsequent PCR targeting the genus Neospora specifically yielded a negative result, corroborating that the consensus DNA sequence obtained by metabarcoding stemmed from Toxoplasma. Efforts to generate T. gondii genotype data from the placental DNA were futile.
Fig. 1.
A, Placental chorionic villi with chronic granulomatous inflammation with multinucleated giant cells (hematoxylin-eosin stain, magnification ×200); B, Chorionic villus with necrotic remnants of Toxoplasma organisms (hematoxylin-eosin stain, magnification ×400). C, Same villus with positive reaction (brown) to Toxoplasma antibody (immunohistochemical stain, magnification ×400). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Serologic and DNA-based methods for immunological work up and detection of infection including Toxoplasma gondii in maternal serum and placenta. Of note, the mother was tested five times over a period of almost one year for Toxoplasma-specific antibodies. Tests performed at the Statens Serum Institut are highlighted in boldface type.
| Analysis | Date of sampling (in chronological order) | Sample material | Result | Note |
|---|---|---|---|---|
| SEROLOGIC ANALYSES | ||||
| Anti-rubella antibodies IgG | December 3, 2020 | Serum | Positive | Positive because of previous infection or immunization |
| Anti-HSV1 IgG antibodies | December 3, 2020 | Serum | Positive | |
| Anti-HSV2 IgG antibodies | December 3, 2020 | Serum | Negative | |
| Anti-T. gondii IgM antibodies | December 8, 2020 | Serum | Negative | |
| Anti-T. gondii IgG antibodies | December 8, 2020 | Serum | Negative | |
| Anti-T. gondiiIgM antibodies | January 26, 2021 | Serum | Negative | |
| Anti-T. gondiiIgG antibodies | January 26, 2021 | Serum | Negative | |
| Anti-T. gondii IgM antibodies | January 26, 2021 | Serum | Negative | |
| Anti-T. gondii IgG antibodies | January 26, 2021 | Serum | Negative | |
| Anti-T. gondii IgM antibodies | February 8, 2021 | Serum | Negative | |
| Anti-T. gondii IgG antibodies | February 8, 2021 | Serum | Negative | |
| Anti-T. gondii IgM antibodies | June 1, 2021 | Serum | Negative | |
| Anti-T. gondii IgG antibodies | June 1, 2021 | Serum | Negative | |
| anti-HSV IgG antibodies | June 1, 2021 | Serum | Positive | |
| anti-rubella IgG antibodies | June 1, 2021 | Serum | Negative | |
| Anti-T. gondiiIgM antibodies | November 29, 2021 | Serum | Negative | |
| Anti-T. gondiiIgG antibodies | November 29, 2021 | Serum | Negative | |
| Total IgG | November 29, 2021 | Serum | 10.7 g/L [6.1–14.9 g/L] | |
| Total IgA | November 29, 2021 | Serum | 2.39 g/L [0.7–4.3 g/L] | |
| Total IgM | November 29, 2021 | Serum | 0.40 g/L [0.39–2.08 g/L] | |
| IgG-subclass group | November 29, 2021 | Serum | NA | |
| IgG1 | November 29, 2021 | Serum | 7.34 g/L [2.8–8.0 g/L] | |
| IgG2 | November 29, 2021 | Serum | 3.27 g/L [1.2–5.7 g/L] | |
| IgG3 | November 29, 2021 | Serum | 1.10 g/L [0.24–1.25 g/L] | |
| IgG4 | November 29, 2021 | Serum | 0.687 g/L [0.052–1.25 g/L] | |
| Anti-Corynebacterium diphtheriaetoxin-specific antibodies | November 29, 2021 | Serum | 1.663 | |
| Anti-Streptococcus pneumoniae-specific IgG antibodies | November 29, 2021 | Serum | Negative⁎ | |
| DNA-BASED ANALYSES | ||||
| Real-time PCR forT. gondii (Homan et al., 2000) | March 10, 2021 | Placental biopsy | Positive | |
| Real-time PCR forTreponema pallidum | March 10, 2021 | Placental biopsy | Negative | |
| Real-time PCR for HSV1, HSV2, and VZV | March 10, 2021 | Placental biopsy | Negative | |
| 18S analysis (metabarcoding) | March 10, 2021 | Placental biopsy | Positive | DNA detected with 100% identity to Apicomplexan parasites including T. gondii and Neospora caninum |
| Real-time PCR forT. gondii (B1 gene) (Slany et al., 2019) | March 10, 2021 | Placental biopsy | Positive | |
| Conventional PCR using the primers used for amplification of the 529-bp fragment | March 10, 2021 | Placental biopsy | Positive | T. gondii-specific DNA sequence obtained (GenBank accession no. OM630157) |
| Conventional PCR forNeospora caninum (McInnes et al., 2006) | March 10, 2021 | Placental biopsy | Negative |
HSV, herpes simplex virus; Ig, immunoglobulin; NA, not applicable; PCR, polymerase chain reaction; VZV, varicella zoster virus.
The woman had not been vaccinated against pneumococci. Tests highlighted in bold-face were carried out at Statens Serum Institut.
No blood or tissue samples from the infant were available for analysis.
Conspicuously, the mother tested negative for immunoglobulin (Ig)M and IgG antibodies against Toxoplasma on several validated tests against a range of antigens. Seronegativity was confirmed 10 months later (Table 1). The serology tests for Toxoplasma used the anti-Toxoplasma IgG and IgM with the VIDAS (bioMérieux) and the ISAGA IgM (BioMérieux) tests.
The mother had serum antibodies against rubella and herpes simplex virus, suggesting immunocompetence. After informed consent, the mother subsequently underwent focused examination for immunocompetence, which indicated that she was able to mount an antibody response against infectious agents (Table 1).
Suspecting an ascending infection and that the fetus might have been infected through semen, the father was tested for anti-T. gondii-specific antibodies; the result indicated that the father had not been exposed to T. gondii (data not shown).
3. Discussion
Congenital toxoplasmosis (CT) typically develops after in-utero infection of a fetus due to vertical transmission of Toxoplasma from the mother. In this case, we observed severe villitis in the presence of Toxoplasma organisms in the placenta belonging to a baby that died four days after birth due to multi-organ failure, which could suggest delayed-onset severe neonatal CT (Al-Hamod et al., 2010). However, the apparently immunocompetent mother was seronegative as confirmed by five serologic workups performed pre- and post-delivery, and so CT in the usual sense was unlikely. This situation prompted speculations on other ways of transmission. The preterm delivery could be due to cervical insufficiency, since the woman had exposed membranes upon arrival in the labor ward. In such cases, the cervical plug is less efficient in preventing ascending infections of the fetus. In line with our hypothesis of delayed severe neonatal toxoplasmosis, and since Toxoplasma may be found in semen (Hlavacova et al., 2021), we speculated that the fetus might have become infected by the father, facilitated by the mother's cervical insufficiency. However, due to the negative paternal anti-T. gondii-specific antibody test, in-utero infection with semen as the vehicle appeared unlikely.
Unfortunately, no samples were available from the infant and therefore the diagnosis of CT remains unconfirmed, although plausible.
As one alternative explanation, the multi-organ failure could have been due to extensive T. gondii-induced placental damage and severe asphyxia in a very preterm infant (Freeman et al., 2005).
In a study of 200 aborting women in Iran, Matin et al. reported the presence of T. gondii-specific DNA in 21 (10.5%) placentas; for four of the 21 women with PCR-positive placentas, no anti-T. gondii-specific immunoglobulins could be detected (Matin et al., 2017). That study was limited by the fact that only one PCR method was used with no histologic examination and DNA sequencing included to confirm the results (i.e., results were based merely on gel visualization of PCR products obtained by nested conventional PCR, which could question the presence of placentitis and the species identification); moreover, follow-up serology data for these women were not reported; hence, it is not known whether these women seroconverted at a later stage.
Meanwhile, a great advantage of the present study was the thorough serological workup (Table 1).
In conclusion, this case of preterm delivery with fatal neonatal outcome illustrates that placental infection with Toxoplasma can take place in the absence of maternal seroconversion, suggesting that placental infection may occur without systemic infection of the mother. If our findings can be corroborated, they may have important implications for ante- and neonatal care, depending on the frequency with which such infections take place.
Disclosures
No funding source was available for this study. None of the authors have any conflicts of interest to declare.
Funding sources
None.
Ethical approval statement
The patient consented to having her data published.
Declaration of Competing Interest
None.
References
- Al-Hamod D., Vauloup C., Goulet M., Zupan-Simunek V., Castel C., Boileau P. Delayed onset of severe neonatal toxoplasmosis. J. Perinatol. 2010;30(3):231–232. doi: 10.1038/jp.2009.184. [DOI] [PubMed] [Google Scholar]
- Armstrong L., Isaacs D., Evans N. Severe neonatal toxoplasmosis after third trimester maternal infection. Pediatr. Infect. Dis. J. 2004;23(10):968–969. doi: 10.1097/01.inf.0000137573.08226.55. [DOI] [PubMed] [Google Scholar]
- Dunay I.R., Gajurel K., Dhakal R., Liesenfeld O., Montoya J.G. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018;31(4) doi: 10.1128/CMR.00057-17. e00057–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K., Oakley L., Pollak A., Buffolano W., Petersen E., Semprini A.E., et al. Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG. 2005;112(1):31–37. doi: 10.1111/j.1471-0528.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- Hlavacova J., Flegr J., Rezabek K., Calda P., Kankova S. Association between latent toxoplasmosis and fertility parameters of men. Andrology. 2021;9(3):854–862. doi: 10.1111/andr.12969. [DOI] [PubMed] [Google Scholar]
- Homan W.L., Vercammen M., De Braekeleer J., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000;30(1):69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Matin S., Shahbazi G., Namin S.T., Moradpour R., Feizi F., Piri-Dogahe H. Comparison of placenta PCR and maternal serology of aborted women for detection of Toxoplasma gondii in Ardabil, Iran. Korean J. Parasitol. 2017;55(6):607–611. doi: 10.3347/kjp.2017.55.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes L.M., Ryan U.M., O’Handley R., Sager H., Forshaw D., Palmer D.G. Diagnostic significance of Neospora caninum DNA detected by PCR in cattle serum. Vet. Parasitol. 2006;142(3–4):207–213. doi: 10.1016/j.vetpar.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Robinson E., de Valk H., Villena I., Le Strat Y., Tourdjman M. National perinatal survey demonstrates a decreasing seroprevalence of Toxoplasma gondii infection among pregnant women in France, 1995 to 2016: impact for screening policy. Euro Surveill. 2021;26(5):1900710. doi: 10.2807/1560-7917.ES.2021.26.5.1900710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slany M., Dziedzinska R., Babak V., Kralik P., Moravkova M., Slana I. Toxoplasma gondii in vegetables from fields and farm storage facilities in the Czech Republic. FEMS Microbiol. Lett. 2019;366(14):fnz170. doi: 10.1093/femsle/fnz170. [DOI] [PubMed] [Google Scholar]
- Stensvold C.R., Jirku-Pomajbikova K., Tams K.W., Jokelainen P., Berg R., Marving E., et al. Parasitic intestinal protists of zoonotic relevance detected in pigs by metabarcoding and real-time PCR. Microorganisms. 2021;9(6):1189. doi: 10.3390/microorganisms9061189. [DOI] [PMC free article] [PubMed] [Google Scholar]