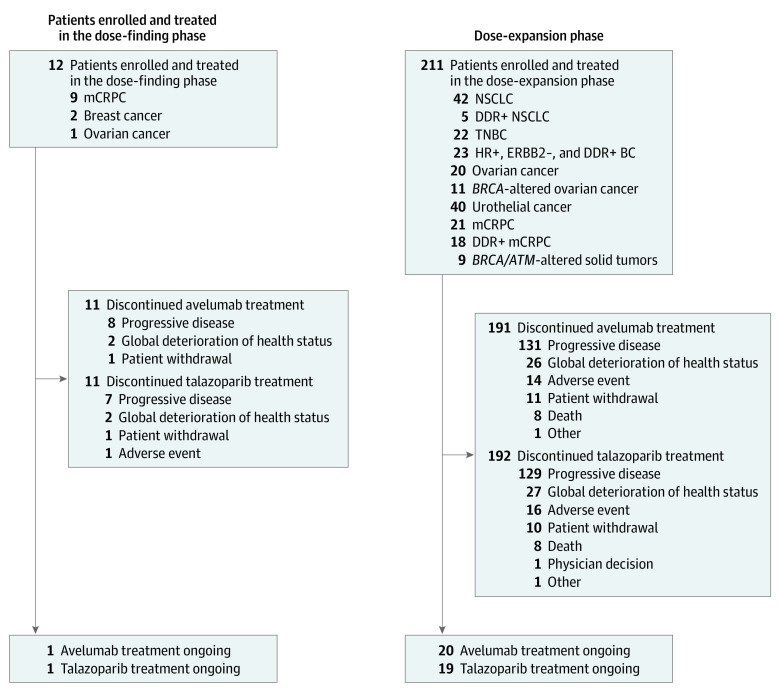
Figure 1. Trial Profile.
In total, 3 patients in the phase 1b portion experienced dose-limiting toxic effects leading to dose interruption. Of them, 2 patients (with thrombocytopenia) continued therapy following talazoparib dose reductions and recovery of platelet counts. Both patients subsequently discontinued from study treatment because of progressive disease; 1 patient discontinued 2 months after the start of treatment, and 1 withdrew from the study after 10 months of treatment. Only 1 patient permanently discontinued talazoparib because of neutropenia (after approximately 3 months); however, this patient continued to receive avelumab for a total of 5 months before discontinuing because of progressive disease. DDR indicates DNA damage repair; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; mCRPC, metastatic castration-resistant prostate cancer; NSCLC, non–small-cell lung cancer; and TNBC, triple-negative breast cancer.