Figure 2. Best Percentage Change in Size of Target Lesions Assessed by Investigators per RECIST v1.1 While Receiving Treatment in the Dose-Expansion Phase.
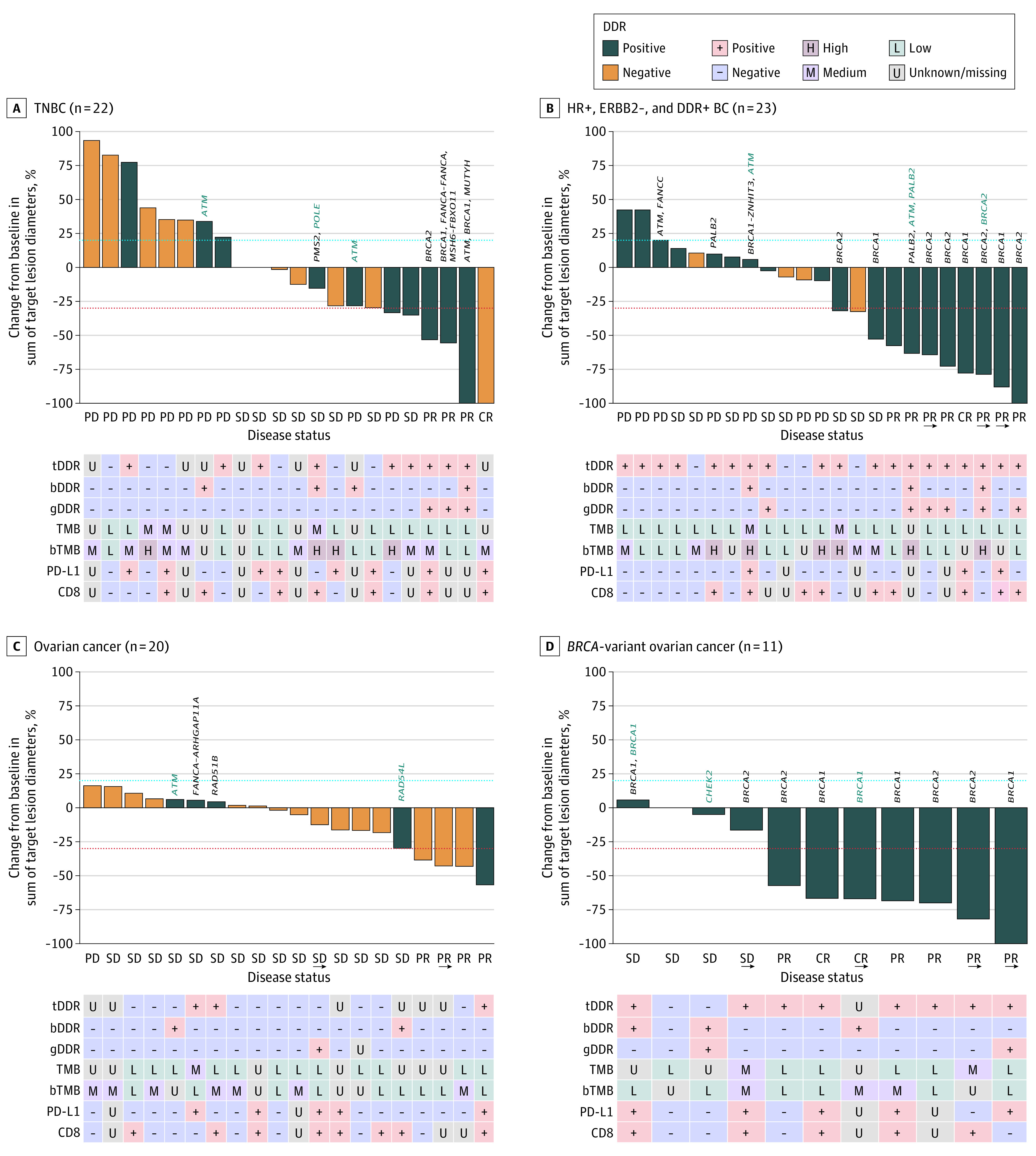
The blue dashed lines represent the threshold for progressive disease (PD), defined as an increase of at least 20% in target lesion diameter from baseline. The lower dashed lines represent the threshold for a partial response, defined as a decrease of at least 30% in target lesion diameter from baseline. In patients with DNA damage repair–positive (DDR+) status but for whom a DDR alteration is not specified, DDR status was confirmed by germline loss of heterozygosity (gLOH) score. Presence of a germline DDR+ alteration (gDDR) alone did not confirm DDR+ status. In the absence of positive solid tumor or circulating tumor DNA alteration results, detection of a known or likely deleterious germline variant suggested that a patient had a DDR+ tumor, provided that solid tumor and circulating tumor DNA results did not both suggest DDR-negative (DDR−). (Three patients were considered to have DDR+ tumors at enrollment, determined by a gLOH score above the predefined cutoff; however, their tumors were subsequently considered DDR− because of a change in gLOH assay specifications. Two patients were enrolled based on a local test result but received negative results centrally.) Arrows indicate ongoing treatment; bDDR, blood DDR; bTMB, blood tumor mutational burden; ERBB2, human epidermal growth factor receptor 2; HR, hormone receptor; PD-L1, programmed cell death 1 ligand 1; SD, stable disease; tDDR, tumor DDR. For some items, the genetic variation is written in vertical text above the bar, with black font indicating tDDR alteration and teal font, bDDR alteration.
