Key Points
Question
Are machine learning–based estimates of tumor-infiltrating lymphocytes (TILs), from hematoxylin-eosin images, associated with response to immune checkpoint inhibitors (ICIs) in patients with non–small cell lung cancer (NSCLC)?
Findings
This retrospective machine learning–based analysis of hematoxylin-eosin digital images of a cohort of patients with NSCLC treated with single-agent ICIs found that higher levels of TILs were associated with higher response rates and improved progression-free and overall survival; in patients with programmed death ligand-1 (PD-L1)-negative cancers, TILs were superior to tumor mutational burden (TMB) in predicting ICI response.
Meaning
In advanced NSCLC, digital scoring of TILs may be clinically useful in predicting benefit from ICI therapy.
This cohort study examines the accuracy of using machine learning–based estimates of tumor-infiltrating lymphocytes from hematoxylin-eosin images to predict response to immune checkpoint inhibitors in patients with non–small cell lung cancer.
Abstract
Importance
Currently, predictive biomarkers for response to immune checkpoint inhibitor (ICI) therapy in lung cancer are limited. Identifying such biomarkers would be useful to refine patient selection and guide precision therapy.
Objective
To develop a machine-learning (ML)-based tumor-infiltrating lymphocytes (TILs) scoring approach, and to evaluate TIL association with clinical outcomes in patients with advanced non–small cell lung cancer (NSCLC).
Design, Setting, and Participants
This multicenter retrospective discovery-validation cohort study included 685 ICI-treated patients with NSCLC with median follow-up of 38.1 and 43.3 months for the discovery (n = 446) and validation (n = 239) cohorts, respectively. Patients were treated between February 2014 and September 2021. We developed an ML automated method to count tumor, stroma, and TIL cells in whole-slide hematoxylin-eosin–stained images of NSCLC tumors. Tumor mutational burden (TMB) and programmed death ligand-1 (PD-L1) expression were assessed separately, and clinical response to ICI therapy was determined by medical record review. Data analysis was performed from June 2021 to April 2022.
Exposures
All patients received anti–PD-(L)1 monotherapy.
Main Outcomes and Measures
Objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were determined by blinded medical record review. The area under curve (AUC) of TIL levels, TMB, and PD-L1 in predicting ICI response were calculated using ORR.
Results
Overall, there were 248 (56%) women in the discovery cohort and 97 (41%) in the validation cohort. In a multivariable analysis, high TIL level (≥250 cells/mm2) was independently associated with ICI response in both the discovery (PFS: HR, 0.71; P = .006; OS: HR, 0.74; P = .03) and validation (PFS: HR = 0.80; P = .01; OS: HR = 0.75; P = .001) cohorts. Survival benefit was seen in both first- and subsequent-line ICI treatments in patients with NSCLC. In the discovery cohort, the combined models of TILs/PD-L1 or TMB/PD-L1 had additional specificity in differentiating ICI responders compared with PD-L1 alone. In the PD-L1 negative (<1%) subgroup, TIL levels had superior classification accuracy for ICI response (AUC = 0.77) compared with TMB (AUC = 0.65).
Conclusions and Relevance
In these cohorts, TIL levels were robustly and independently associated with response to ICI treatment. Patient TIL assessment is relatively easily incorporated into the workflow of pathology laboratories at minimal additional cost, and may enhance precision therapy.
Introduction
Programmed cell death-1 and/or programmed death ligand-1 (PD-1/PD-L1) pathway–targeted immunotherapy has revolutionized the treatment of multiple malignant diseases.1 For advanced-stage non–small cell lung cancer (NSCLC), the combination of chemotherapy with immune checkpoint inhibitor (ICI) therapy has become the standard treatment for most patients. However, less than half of all patients with NSCLC benefit from anti-PD-(L)1 therapies as a single agent.2,3 Favorable ICI treatment responses in NSCLC have been associated with high-level PD-L1 expression, high tumor mutation burden (TMB), microsatellite instability, and tumor-inflamed phenotype.4
However, none of these measures are absolute predictors of ICI response, and identification of additional biomarkers of response has clear value. Tumor-infiltrating lymphocytes (TILs) have been studied for several decades,5,6 and high levels have been reported as a favorable prognostic biomarker,7,8 and to be associated with immunotherapy response in patients with NSCLC.9 The standard method for evaluating TILs is based on routine hematoxylin-eosin–stained slides using a semiquantitative scoring method, which is subjective and therefore not highly reproducible.10 Image processing and computational methods potentially enable rapid, reproducible, and quantitative analysis of biomedical images. In particular, artificial intelligence (AI)-based computational pathology has recently shown promise in predicting the tissue of origin for unknown primary tumors,11 mutational status,12 and tumor grading.13
Artificial intelligence–based models can analyze complex spatial patterns, and overcome limitations associated with manual scoring and human bias. Preliminary studies have shown that such algorithms are able to quantify TILs on hematoxylin-eosin–stained slides.14,15 We extended these studies by development of a machine-learning (ML) based algorithm for quantification of TILs on hematoxylin-eosin–stained sections in NSCLC that operates with minimal user intervention. We then examined association of TIL levels as a quantitative variable with clinical outcomes in a large series of patients with advanced NSCLC treated with ICI monotherapy. To validate the findings, we analyzed an independent cohort of patients with advanced NSCLC. We also examined the relative classification performance of TMB, PD-L1, and TIL levels, for ICI treatment response.
Methods
Patients and Pathology Materials
The initial study began with 551 consecutive patients diagnosed with advanced NSCLC at Dana-Farber Cancer Institute (DFCI). These patients all had OncoPanel tumor mutation analysis, and had been treated with PD-(L)1 inhibitors. Patients with cytological samples only were excluded (n = 105 [19%]), leaving 446 patients for analysis. The DFCI cohort was analyzed as a discovery cohort to develop the ML model and to define the cutoff for TILs density. An independent validation cohort of 239 patients with NSCLC who had received anti–PD-(L)1 treatment at the Imperial College of London (ICL, n = 78) in the UK, and at the Amsterdam University Medical Center (UMC, n = 161) in the Netherlands were also studied. This study was approved by the institutional review board at each center and written informed consent was waived because all data were deidentified.
Procedures
Tumor mutational burden was calculated from the DFCI OncoPanel targeted sequencing platform as previously described16; PD-L1 expression was assessed using validated anti-PD-L1 antibodies as part of routine clinical care.17 Using supervised machine learning algorithms (QuPath v.0.2.3),18 image analysis was performed on whole-slide hematoxylin-eosin images of the biopsy and resected samples (excluding artifacts and necrosis) except for metastatic lymph nodes (LN). In LN tissues, the preexisting lymphoid stroma were excluded and the tumor nest and desmoplastic stromal areas only were considered for analysis, based on the International Immuno-Oncology working group guideline19 (eFigure 1 in the Supplement). The workflow and an example of the analysis output are shown in eFigure 13 in the Supplement. Participant TIL levels were treated as a categorial variable for analysis using an optimal cutoff.20 The optimal cutoff was determined by the log-rank maximization method as implemented by Hothorn et al21 in MaxStat R package (R Project for Statistical Computing). Detailed methods are reported in the eMethods in the Supplement.
Results
TILs and Clinical Parameters
A total of 685 patients with advanced-stage NSCLC who were treated with first- or subsequent-line ICI monotherapy were studied from 2 independent cohorts. Baseline patient and tumor characteristics (Table) of the discovery and validation cohorts were overall similar and were typical of a population with metastatic NSCLC. However, the validation cohort had a lower proportion of patients with resected material (47 of 239 [20%] vs 252 of 446 [57%]), PD-L1 high (≥50%) expression (87 of 239 [36%] vs 173 of 446 [52%]), adenocarcinoma histologic findings (158 of 239 [67%] vs 352 of 446 [79%]), and female sex (97 of 239 [41%] vs 248 of 446 [56%]) compared with the discovery cohort. The median (range) age in both cohorts was 66 (30-94) years. Nearly all patients received anti-PD-1 therapy with pembrolizumab (363 [53%]) or nivolumab (270 [39%]); 42 (6%) received anti–PD-L1 therapy with atezolizumab.
Table. Baseline Characteristics of the Discovery and Validation NSCLC Cohorts Treated With ICI Monotherapy.
| Characteristic | No. (%) | P valuea | Total [n = 685], No. (%) | |
|---|---|---|---|---|
| Discovery cohort (n = 446) | Validation cohort (n = 239) | |||
| Treatment agent | ||||
| Pembrolizumab | 246 (55) | 117 (49) | .20 | 363 (53) |
| Nivolumab | 164 (37) | 106 (44) | 270 (39) | |
| Atezolizumab | 28 (6) | 14 (6) | 42 (6) | |
| Otherb | 8 (2) | 2 (1) | 10 (2) | |
| ICI line | ||||
| 1 | 179 (40) | 91 (38) | .60 | 270 (39) |
| ≥2 | 267 (60) | 148 (62) | 415 (61) | |
| Age | ||||
| <66 | 221 (49) | 119(50) | .90 | 340 (49) |
| ≥66 | 225 (51) | 120 (50) | 345 (51) | |
| Sex | ||||
| Female | 248 (56) | 97 (41) | <.001 | 345 (51) |
| Male | 198 (44) | 142 (59) | 340 (49) | |
| Histologic findings | ||||
| LUAD | 352 (79) | 158 (67) | .002 | 510 (75) |
| LUSC | 64 (14) | 55 (23) | 119 (17) | |
| Other | 30 (7) | 23 (10) | 53 (8) | |
| Smoking | ||||
| Never | 58 (13) | 25 (10) | .30 | 83 (13) |
| Ever | 388 (87) | 208 (87) | 596 (86) | |
| Unknown | 0 | 6 (3) | 6 (1) | |
| ECOG | ||||
| 0-1 | 362 (81) | 197 (82) | .30 | 559 (82) |
| ≥2 | 82 (19) | 36 (15) | 118 (17) | |
| Unknown | 2 (<1) | 6 (3) | 8 (1) | |
| Specimen site | ||||
| Lung | 224 (50) | 124 (52) | .20 | 348 (51) |
| Lymph node | 60 (13) | 32 (13) | 92 (13) | |
| Pleura | 41 (9) | 11 (5) | 52 (8) | |
| Brain | 37 (8) | 7 (3) | 44 (6) | |
| Liver | 27 (6) | 23 (10) | 50 (7) | |
| Soft tissue | 27 (6) | 15 (6) | 42 (5) | |
| Otherc | 30 (8) | 27 (11) | 57 (8) | |
| Tumor type | ||||
| Primary | 202 (45) | 122 (51) | .20 | 324 (48) |
| Metastatic | 233 (53) | 117 (49) | 350 (51) | |
| Unknown | 11 (2) | 0 | 11 (1) | |
| Tissue type | ||||
| Resection | 252 (57) | 47 (20) | <.001 | 299 (44) |
| Biopsy | 194 (43) | 192 (80) | 386 (56) | |
| KRAS/EGFR status | ||||
| KRAS | 164 (37) | 73 (31) | .50 | 237 (35) |
| EGFR | 38 (9) | 5 (2) | 43 (6) | |
| KRAS/EGFR neg | 241 (54) | 117 (49) | 358 (52) | |
| Not tested | 3 (<1) | 44 (18) | 47 (7) | |
| PD-L1, TPS % | ||||
| <1 | 50 (15) | 66 (27) | <.001 | 116 (17) |
| 1-49 | 111 (33) | 40 (16) | 151 (22) | |
| ≥50 | 173 (52) | 87 (36) | 260 (38) | |
| Unknown | 112 (25) | 46 (19) | 158 (23) | |
| TMB, mu/Mb | ||||
| <10 | 227 (51) | NA | NA | 227 (51) |
| ≥10 | 217 (49) | NA | 217 (49) | |
| Unknown | 2 (<1) | NA | 2 (<1) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitors; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; mu/Mb, mutations per megabase; NA, not assessed; NSCLC, non-small cell lung cancer; PD-L1, programmed death ligand-1; TMB, tumor mutational burden; TPS, tumor proportion score.
P values were based on χ2 test.
Including durvalumab and commercial immunotherapy agents.
Including adrenal, breast, kidney, skin, stomach, oral, and small intestine.
The distribution of dichotomized (≥ vs <250 cells/mm2, optimal cutoff) TIL levels by baseline clinical attributes was investigated (eTable 1 in the Supplement). Overall, TIL levels showed a significant association with tumor type (primary vs metastatic) and specimen site in the discovery cohort. In the validation cohort, there was no significant correlation between TIL subgroups and the clinicopathologic variables except for treatment agent and ICI line of therapy.
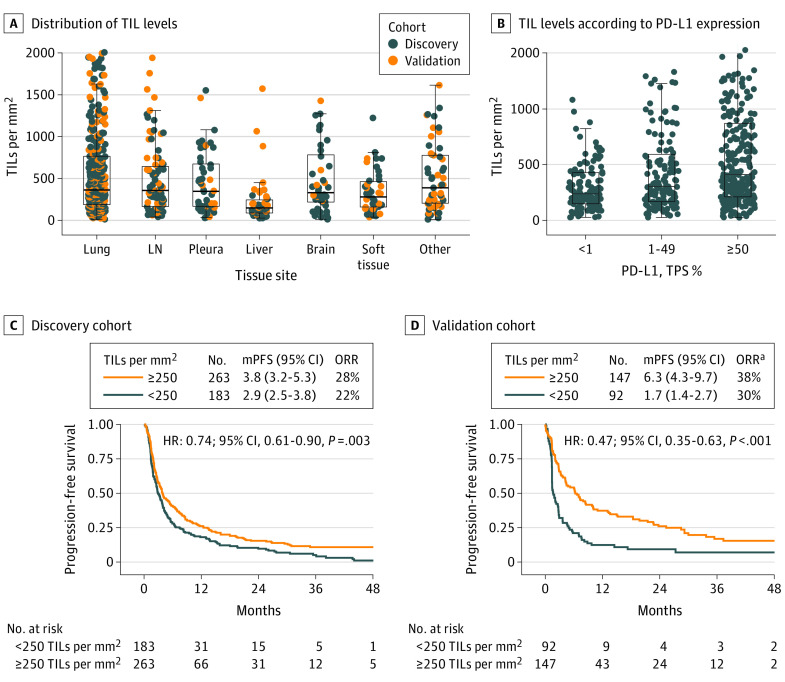
The ML output on cell subset measurements is shown in eTable 2 in the Supplement. Tumor percent was a median (IQR) of 65% (47%-80%). To evaluate the performance of the ML model, we compared the ML estimate of tumor cell prevalence with manual and postsequencing tumor cellularity estimates in the DFCI cohort. Even though the pathologist tumor percentage estimate is a subjective assessment, we found a significant and moderate intraclass correlation coefficient (ICC) between manual vs ML-based tumor cellularity estimations across all tissue sites, whereas the ICC was poor for lymph node and others (eFigure 2 in the Supplement). Overall, TIL levels were highly variable, with a median (IQR) of 327 (164-681) cells/mm2. Participant TIL levels were assessed on biopsy or resected samples from various tissue sites, and were similar across all sites except for metastatic liver lesions, for which median TIL levels were 148 cells/mm2 (n = 50) (Figure 1A; eFigure 3 in the Supplement).
Figure 1. Tumor-Infiltrating Lymphocytes (TILs) Distribution and Clinical Outcome.
A, Distribution of TIL levels (count per mm2) across different tissue sample sites (Kruskal-Wallis test, P < .001) in both discovery and validation cohorts. B, TIL levels according to PD-L1 expression (P = .01, Kruskal-Wallis test) in the entire cohort. C, Progression-free survival (PFS) following treatment with immune checkpoint inhibitors for patients with <250 vs ≥250 TILs per mm2 in the discovery (C) and (D) validation cohorts. HR indicates hazard ratio; mPFS, median PFS in months; ORR, objective response rate; PD-L1, programmed death ligand-1; TPS, tumor proportion score.
aORR was available for only 101 out of 239 patients in the validation cohort.
In the discovery cohort, the median (IQR) TMB was 9.9 (6.8-13.7) mutations per megabase (mu/Mb), and there was no correlation between TMB and TIL levels or between TMB and PD-L1 expression (eFigure 4 in the Supplement). There was an association between PD-L1 expression and TIL levels (eFigure 4 in the Supplement), with somewhat higher TIL levels (cells/mm2) seen in patients with PD-L1 expression of 50% or greater (median = 410) compared with the PD-L1 low (<1%, median = 238) or intermediate subgroups (1%-49%, median = 303; Figure 1B; P = .01).
We analyzed the association between TIL levels and potentially targetable driver cancer gene mutations seen at more than 5% frequency (KRAS, EGFR). Importantly, there was no difference in TIL levels in KRAS-mutant vs KRAS wild-type lung adenocarcinoma. In contrast, TILs/mm2 levels were significantly lower in EGFR-mutant (median = 164) compared with EGFR wild-type adenocarcinoma (median = 327, eFigure 5 in the Supplement).
TILs and Clinical Outcomes
The median (IQR) follow-up of the patients was 38.1 (26.3-49.2) and 43.3 (28.1-54.8) months, and the median (IQR) progression-free survival (PFS) was 3.5 (1.6-10.1) and 3.6 (1.4-15.2) months for the discovery and validation cohorts, respectively. In the discovery cohort, after stratification of patients based on TIL levels (≥ vs <250 cells/mm2; eMethods, eFigure 6 in the Supplement), patients with high TIL levels had a significantly longer median PFS (3.8 vs 2.9 months; HR = 0.74; P = .003, Figure 1C), and a significantly longer overall survival (OS, 15.5 vs 11.8 months; HR = 0.76; P = .02, eFigure 7 in the Supplement). Using the TIL optimal cutoff defined in the discovery cohort, we confirmed the association of high TIL levels with clinical outcomes in the validation cohort: there was longer PFS for patients with high TILs (6.3 vs 1.7 months; HR = 0.47; P < .001; Figure 1D), and longer OS (13.1 vs 5.7 months; HR = 0.50, P < .001; eFigure 7 in the Supplement). Importantly, the derived cutoff for TIL levels (≥ vs <250 cells/mm2) in the discovery cohort was very similar to that of the validation cohort (293 cells/mm2), and was exactly the same in the combined cohorts (251 cells/mm2), determined using the maximally selected log-rank statistic (eFigure 6 in the Supplement).
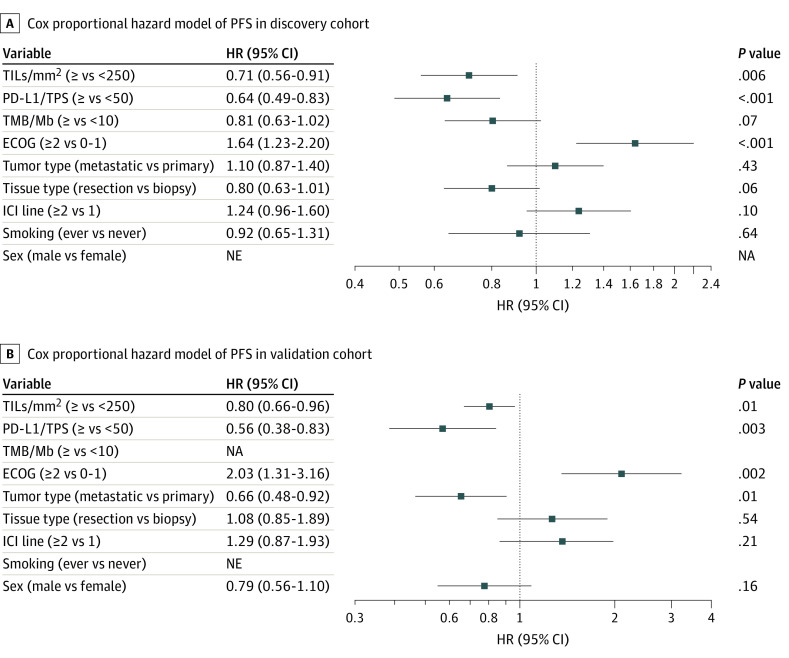
In addition to TILs, several clinical variables also showed an association with benefit to ICI monotherapy (defined as P < .25 for either PFS or OS) in univariate analysis (eTable 3 in the Supplement). Significant covariates were similar in both the discovery and validation cohorts except for smoking and sex. In the multivariable analysis of the discovery cohort, after adjusting for covariates, TIL levels were independently associated with both PFS (HR = 0.71; P = .006; Figure 2A) and OS (HR = 0.74; P = .03; eFigure 8 in the Supplement). Similar findings were observed for the validation cohort, in which the association of TIL levels with PFS (HR = 0.80; P = .01; Figure 2B) and OS (HR = 0.75; P = .001; eFigure 8 in the Supplement) remained statistically significant after controlling for covariates.
Figure 2. Forest Plots for Progression-Free Survival (PFS).
Forest plot of hazard ratio (HR) and 95% CI for PFS according to covariates in the (A) discovery (n= 446) and (B) validation (n=239) cohorts. In addition to TIL density (<250 vs ≥250 cells/mm2), clinicopathologic variables with P < .25 from univariate analyses were included. ECOG, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitors; NA, not assessed; NE, not entered; PD-L1, programmed death ligand-1; TMB, tumor mutational burden; TPS, tumor proportion score.
In the discovery cohort, of the 446 patients evaluable for efficacy, the objective response rate (ORR), including complete or partial response, was 26% (n = 114). In the validation cohort, ORR was collected for 101 of 239 patients, in which 35% (n = 35) were responders. In the entire cohort with available ORR (n = 547), using TIL levels as a quantitative variable, patients who experienced a partial/complete response had a trend to higher median TIL/mm2 compared with those who had progressive/stable disease (384 vs 323, P = .09, eFigure 9 in the Supplement). Although a similar trend was also found in patients with high (≥250) compared with low (<250) TIL/mm2 levels with ORR of 28% vs 22%, and 38% vs 30% for discovery and validation cohorts, respectively, neither of these reached statistical significance (2-sided χ2 = 0.2; 0.3, respectively, eFigure 9 in the Supplement).
In the combined cohort, most patients were treated with second- or subsequent-line ICI monotherapy (n = 415). In the subgroup analysis based on the ICI treatment line, there was a statistically significant association between TIL levels and response for both first-line treatment (PFS: 7.1 vs 3.7 months; HR = 0.52; P < .001; OS: 24.3 vs 13.3 months; HR = 0.59; P < .001) and for second-line treatment or beyond (PFS: 3.2 vs 2.3 months; HR = 0.76; P = .008; OS: 11.9 vs 6.9 months; HR = 0.77; P = .01) (eFigure 10 in the Supplement).
Relative Significance of Different Biomarkers in the Association With ICI Response
The validation cohort did not have TMB data values. Therefore, classification models were constructed using the discovery cohort only. To explore the relative contribution of different biomarkers in associating ORR with ICI treatments, receiver operating curve analysis was performed including TIL levels, PD-L1, and TMB. Considering single biomarkers, PD-L1 had the highest area under the curve (AUC) (0.68, P < .001), whereas TIL levels (AUC = 0.55, P = .08) and TMB (AUC = 0.59, P = .05) levels had lower AUC values for classifying responders from nonresponders (Figure 3A; eTable 4 in the Supplement).
Figure 3. Combined Models.
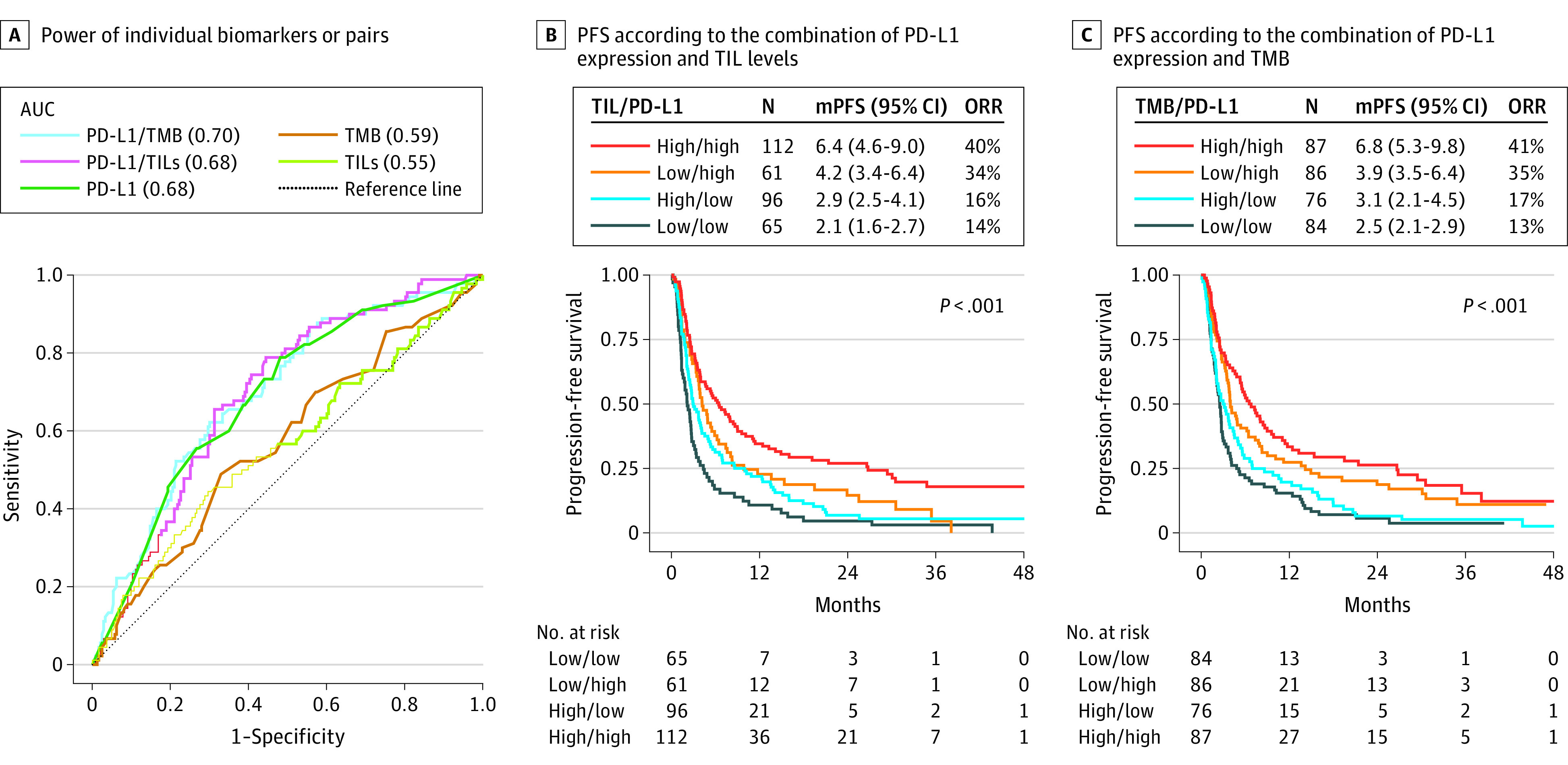
A, Power of individual biomarkers (programmed death ligand-1 [PD-L1], tumor mutational burden [TMB], tumor-infiltrating lymphocytes [TILs]) or pairs with PD-L1 to predict immune checkpoint inhibitor response rate computing area under the receiver operating characteristic curve. B, Progression-free survival according to the combination of PD-L1 (<50 vs ≥50% ) expression and hematoxylin-eosin TIL levels (<250 vs ≥250 cells/mm2) scores. C, Progression-free survival according to the combination of PD-L1 (<50 vs ≥50%) expression and TMB (<10 vs ≥10 mu/Mb). mPFS indicates median PFS in months; ORR, objective response rate.
Combining these biomarkers using weighted linear regression, PD-L1/TMB had the greatest AUC (0.70, P < .001). Both PD-L1/TIL and PD-L1/TMB showed an improved specificity estimate value (0.56 and 0.70, respectively) compared with PD-L1 alone (0.52). In addition, the combined assays, PD-L1/TMB or PD-L1/TIL, had higher positive predictive value (PPV) compared with single assays (eTable 4 in the Supplement). These findings were also supported by survival analysis, which showed that the high/high (TILs ≥250 cells/mm2; PD-L1 ≥ 50%) subset had the best PFS and highest (45 of 112 [40%]) ORR, while the low/low group had the worst PFS and lowest (9 of 65 [14%]) ORR (Figure 3B). This observation was confirmed using OS as the endpoint (eFigure 11 in the Supplement). A similar pattern of survival benefit was observed for combined TMB/PD-L1 (Figure 3C; eFigure 11 in the Supplement).
Prediction of ICI Response in PD-L1 Subgroups
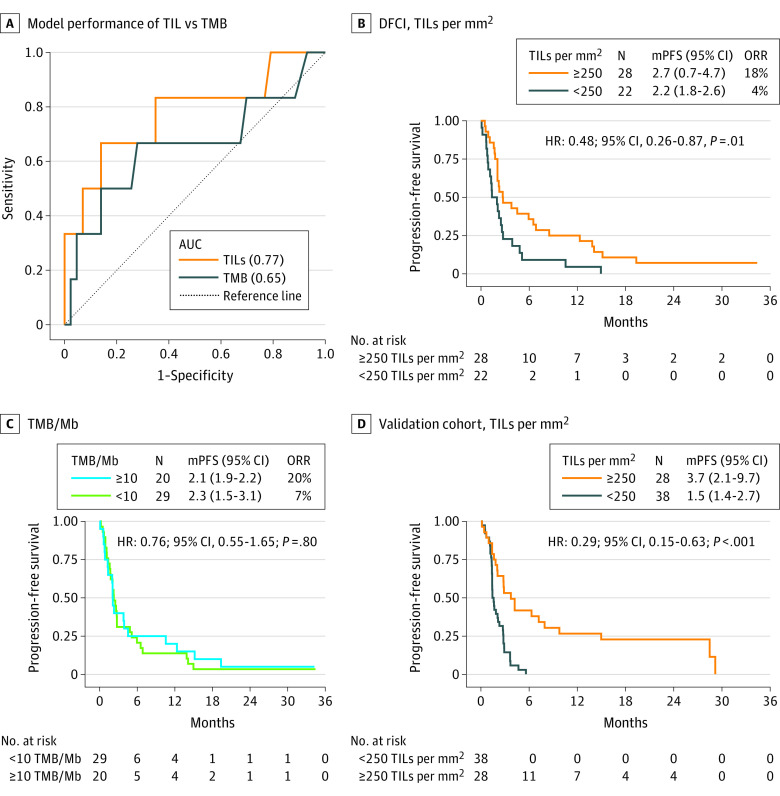
We next stratified our cohort based on PD-L1 expression status to assess whether TMB or TIL levels had classification accuracy for ICI responses across different levels of PD-L1 expression in the discovery cohort. In PD-L1–negative (<1%) patients, TIL levels had an AUC of 0.77 (P = .02) and PPV of 0.40, whereas TMB had an AUC of 0.65 (P = .30) and PPV of 0.25, for the classification of responders from nonresponders (Figure 4A; eTable 4 in the Supplement). Similarly, only 1 of 22 (4%) were responders in the low TIL level (<250/mm2) subset of PD-L1–negative patients, vs 2 of 29 (7%) in the low TMB (<10/Mb) subset. Furthermore, TIL levels were also significantly associated with improved PFS (Figure 4B) and a positive trend to a better OS in PD-L1–negative patients (eFigure 12 in the Supplement). No association was observed with TMB in this subgroup (Figure 4C). The survival advantage of TILs in the PD-L1–negative (n = 66) subgroup was also preserved in the validation cohort for both PFS (Figure 4D) and OS (eFigure 12 in the Supplement) end points.
Figure 4. Tumor-Infiltrating Lymphocytes (TILs) and Tumor Mutational Burden (TMB) in Programmed Death Ligand-1 (PD-L1)-Negative Subgroup.
A, Model performance of TIL vs TMB to predict ICI response in the PD-L1–negative (<1%) subgroup (n = 50) of the DFCI cohort. B, Progression-free survival (PFS) to ICIs for TILs/mm2. C, TMB/Mb in PD-L1 negative subgroup of the DFCI cohort. D, PFS to ICIs for TILs/mm2 in the PD-L1–negative subgroup (n = 66) of the validation cohort. AUC indicates area under the curve; DFCI, Dana-Farber Cancer Institute; HR hazard ratio; ICI, immune checkpoint inhibitors; mPFS, median PFS in months; ORR, objective response rate.
In the PD-L1 intermediate (1%-49%) and high (≥50%) subsets, both TILs and TMB had relatively poor classification performance of differentiating responders (eTable 4 in the Supplement). However, in the high PD-L1 subset, TIL levels showed a significant association with PFS in only the discovery cohort (eFigure 12 in the Supplement), whereas high TMB (≥10/Mb) had no survival (PFS, OS) association in both the PD-L1 intermediate and high subsets.
Discussion
To our knowledge, this is the largest study to use an automated procedure, machine learning, to quantify TIL levels in routine hematoxylin-eosin slides of NSCLC tumors. In 2 large and independent US and European-based cohorts of patients with advanced-stage NSCLC, we found a superior outcome (PFS, OS) for patients with higher TIL levels receiving ICI monotherapy, independent of other clinical or molecular biomarkers. In addition, joint biomarker assays of PD-L1/TILs and PD-L1/TMB showed an improved classification performance of ICI response compared to single assays. Furthermore, in the PD-L1–negative subset, we found that TIL levels had higher sensitivity and specificity in identifying ICI responders compared with TMB.
ML and TILs
A series of guidelines have been published by the International Immuno-Oncology working group for manual assessment of TILs in hematoxylin-eosin slides.22,23 Despite these standardization efforts, the subjective nature and degree of variability in TIL level assessment has limited its translational adoption into clinical practice.24 For this reason, we developed supervised ML-based methods to identify and quantify TILs in standard hematoxylin and eosin histologic sections of advanced stage NSCLC using open source software (Qupath).25,26 In our study, the ML models were improved iteratively through a series of quality control steps by experienced pathologists (J.V, E.R, and W.S). We were able to train different classifiers specific for each of 7 distinct tissue types in which metastatic lung cancer had been found on samples (lung, lymph node, pleura, brain, liver, soft tissue, and other). To our knowledge, this is the first study implementing ML models to assess TILs on patients with advanced NSCLC including diverse metastatic tissue types. In a recently published series of 518 whole-tissue image analysis,27 TILs were associated with better ICI outcomes, but the analysis excluded LNs and distant metastatic specimens. Nevertheless, future efforts should address whether a universal machine or deep learning-based classifier could be used independent of tissue type adjustment, or other variables such as different image formats.
TILs Localization
Previous studies have reported the prognostic value of intratumoral, stromal, and total tumor-stroma TILs in NSCLC, with some reports favoring intratumoral localization,27and others total tumor stroma.8 We have previously reported that stromal CD8-positive TILs had superior prognostic value compared with intratumoral TILs in resected and early-stage NSCLC.28 However, biopsy specimens are the only specimens available for many patients with late-stage NSCLC, 56% (386/685) in the current study. Thus, we believe that attempting to distinguish between intratumoral and stromal TIL is problematic and best avoided due to poor tumor-stroma architecture in many biopsy specimens.
TILs vs TMB
Recently, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high TMB (>10 mu/Mb) levels with a variety of solid tumors.29 However, TMB is at best an imperfect predictor of response to ICI therapy.30 Importantly, we saw no correlation between TMB and PD-L1 expression, similar to previous reports.31,32 Furthermore, both TIL levels and TMB counts had relatively poor sensitivity and specificity to predict ICI responders. Furthermore, previous studies have noted increased ORR and improved PFS in patients with both high PD-L1 and high TMB in response to ICI therapy.33,34 A common feature of the available predictive biomarkers to ICI treatment is the high sensitivity, but low specificity because it has been easier to identify responders, but challenging to predict nonresponders. This may be a consequence of clinical and molecular heterogeneity in the nonresponders to immunotherapy.35 Here we found that a combination of high PD-L1 with high TMB led to 18% improved specificity (0.70) compared with PD-L1 assessment alone, with specificity of 0.52. The possibility that combination assessment of PD-L1 and TIL levels or TMB is a superior biomarker for ICI response in patients with NSCLC warrants further validation. In addition, considering the cost associated with TMB estimation, digital TIL scores may be preferable for implementation in routine practice at the minimal cost of an ML analysis.
TILs in PD-L1–Negative Patients
Although high PD-L1 expression clearly enriches for response to PD-(L)1 inhibitors in patients with NSCLC, it is well known that patients with PD-L1–negative status can also respond to such treatment, with response rates as high as 22% (CheckMate 017) to 25% (CheckMate 057).36 In this patient subset, we found that TIL levels were associated with ICI response to a greater extent than TMB. In addition, very few PD-L1–negative patients who also had low TIL levels responded to ICI, whereas this was somewhat higher in the PD-L1–negative TMB-low subset. Thus, detection and quantification of TIL levels may provide important predictive information in this PD-L1–negative subgroup. Nevertheless, these results should be interpreted cautiously and a larger study better powered for the PD-L1–negative subgroup analyses would provide more definitive information.
Limitations and Future Directions
The strength of this study lies in the number of patients, the use of both a discovery and validation cohort, and the analysis of primary, LN, and distant metastatic specimens, which are commonly obtained from patients with NSCLC. Even though the cutoff defined by analysis of the primary cohort performed well in the validation cohort, future prospective studies to validate these findings are desirable. With the increased interest in neoadjuvant and adjuvant therapies using ICIs in patients with earlier-stage NSCLC, ML-based TIL profiling could be explored further in those settings. In addition, using multiplexed immunoprofiling, it is possible to examine whether this survival advantage is due to the one particular subset of the TIL populations (such as B, Th1, Th2, Th17, TFH, cytotoxic T-cells; and functional subgroups of these).37
Conclusions
The findings of this cohort study suggest that TIL levels determined by ML-based methods are associated with clinical benefit from ICI therapy in patients with advanced-stage NSCLC. Overall, TIL levels showed a stronger association with response to ICI therapy than TMB levels in patients with PD-L1–negative NSCLC. Furthermore, TIL levels can be assessed on standard hematoxylin-eosin stained slides, and their quantitative assessment is easily implemented using the ML approach at minimal cost. It is a scalable and cost-effective method with potential widespread translation to routine clinical care.
eFigure 1. Areas included for TILs assessment in LN tissues.
eFigure 2. ML-based tumor content versus manual estimation in DFCI cohort
eFigure 3. Range of TIL density in different sample types.
eFigure 4. Correlation between TIL levels and TMB/PD-L1.
eFigure 5. TIL levels according to KRAS and EGFR mutation status in the discovery cohort.
eFigure 6. Log-rank test cutoff identification
eFigure 7. TIL levels and overall survival to immunotherapy
eFigure 9. TILs and objective response rate (ORR)
eFigure 10. Treatment line-TILs interaction. Progression-free and overall survival in treatment subgroups of the combined cohorts.
eFigure 11. Combined TILs/PD-L1, TMB/PD-L1 and immunotherapy outcome
eFigure 12. TILs and immunotherapy outcome based on PD-L1 stratification
eFigure 13. Quantification of TILs by machine-learning (ML) methods
eTable 1. Patient characteristics according to TIL levels in the discovery and validation cohorts
eTable 2. Machine-learning derived quantitative detail of the cell subsets from the histological H&E images in the entire cohort, including both the discovery and validation cohorts (n = 685)
eTable 3. Clinicopathologic variables, including TILs, in association with PFS and OS to ICIs in A) discovery and B) validation cohorts (Univariate analyses, Log-rank test, unadjusted Cox proportional hazard ratios)
eTable 4. Comparison of different single and multi-assays sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for responders versus non-responders after ICI treatment in overall discovery cohort and PD-L1 subsets. 95% CI in parentheses
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133-150. doi: 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944-5951. doi: 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 6.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959-2966. doi: 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakaee M, Kilvaer TK, Dalen SM, et al. Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non-small cell lung cancer. Hum Pathol. 2018;79:188-198. doi: 10.1016/j.humpath.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 8.Brambilla E, Le Teuff G, Marguet S, et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol. 2016;34(11):1223-1230. doi: 10.1200/JCO.2015.63.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gataa I, Mezquita L, Rossoni C, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer. 2021;145:221-229. doi: 10.1016/j.ejca.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Kos Z, Roblin E, Kim RS, et al. ; International Immuno-Oncology Biomarker Working Group . Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6(1):17. doi: 10.1038/s41523-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu MY, Chen TY, Williamson DFK, et al. AI-based pathology predicts origins for cancers of unknown primary. Nature. 2021;594(7861):106-110. doi: 10.1038/s41586-021-03512-4 [DOI] [PubMed] [Google Scholar]
- 12.Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567. doi: 10.1038/s41591-018-0177-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer. NPJ Digit Med. 2019;2(1):48. doi: 10.1038/s41746-019-0112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Väyrynen JP, Lau MC, Haruki K, et al. Prognostic significance of immune cell populations identified by machine learning in colorectal cancer using routine hematoxylin and eosin-stained sections. Clin Cancer Res. 2020;26(16):4326-4338. doi: 10.1158/1078-0432.CCR-20-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acs B, Ahmed FS, Gupta S, et al. An open source automated tumor infiltrating lymphocyte algorithm for prognosis in melanoma. Nat Commun. 2019;10(1):5440. doi: 10.1038/s41467-019-13043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1(19):e87062. doi: 10.1172/jci.insight.87062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamberti G, Spurr LF, Li Y, et al. Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Ann Oncol. 2020;31(6):807-814. doi: 10.1016/j.annonc.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 18.Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. doi: 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311-335. doi: 10.1097/PAP.0000000000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y, Cole K, Martinez-Morilla S, et al. An open source, automated tumor infiltrating lymphocyte algorithm for prognosis in triple-negative breast cancer. Clin Cancer Res. 2021;27(20):5557-5565. doi: 10.1158/1078-0432.CCR-21-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121-137. doi: 10.1016/S0167-9473(02)00225-6 [DOI] [Google Scholar]
- 22.Dieci MV, Radosevic-Robin N, Fineberg S, et al. ; International Immuno-Oncology Biomarker Working Group on Breast Cancer . Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52(Pt 2):16-25. doi: 10.1016/j.semcancer.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S, et al. ; International TILs Working Group 2014 . The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259-271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Bockstal MR, François A, Altinay S, et al. Interobserver variability in the assessment of stromal tumor-infiltrating lymphocytes (sTILs) in triple-negative invasive breast carcinoma influences the association with pathological complete response: the IVITA study. Mod Pathol. 2021;34(12):2130-2140. doi: 10.1038/s41379-021-00865-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Z, Xu S, Shao W, et al. Deep-learning-based characterization of tumor-infiltrating lymphocytes in breast cancers from histopathology images and multiomics data. JCO Clin Cancer Inform. 2020;4:480-490. doi: 10.1200/CCI.19.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acs B, Pelekanou V, Bai Y, et al. Ki67 reproducibility using digital image analysis: an inter-platform and inter-operator study. Lab Invest. 2019;99(1):107-117. doi: 10.1038/s41374-018-0123-7 [DOI] [PubMed] [Google Scholar]
- 27.Park S, Ock C-Y, Kim H, et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non-small-cell lung cancer. J Clin Oncol. 2022;40(17):1916-1928. doi: 10.1200/JCO.21.02010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnem T, Hald SM, Paulsen E-E, et al. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2635-2643. doi: 10.1158/1078-0432.CCR-14-1905 [DOI] [PubMed] [Google Scholar]
- 29.Subbiah V, Solit DB, Chan TA, Kurzrock R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians. Ann Oncol. 2020;31(9):1115-1118. doi: 10.1016/j.annonc.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27(5):1236-1241. doi: 10.1158/1078-0432.CCR-20-3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devarakonda S, Rotolo F, Tsao MS, et al. Tumor mutation burden as a biomarker in resected non-small-cell lung cancer. J Clin Oncol. 2018;36(30):2995-3006. doi: 10.1200/JCO.2018.78.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariam A, Kamath S, Schveder K, McLeod HL, Rotroff DM. Large-scale meta-analysis of potential biomarkers for treatment response to anti-PD-1/PD-L1 immune checkpoint inhibitors. medRxiv. November 2020:2020.11.25.20238865. doi: 10.1101/2020.11.25.20238865 [DOI] [Google Scholar]
- 34.Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195-1204. doi: 10.1001/jamaoncol.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricciuti B, Arbour KC, Lin JJ, et al. Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol. 2022;17(3):399-410. doi: 10.1016/j.jtho.2021.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. doi: 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sholl LM. Biomarkers of response to checkpoint inhibitors beyond PD-L1 in lung cancer. Mod Pathol. 2022;35(July)(suppl 1):66-74. doi: 10.1038/s41379-021-00932-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Areas included for TILs assessment in LN tissues.
eFigure 2. ML-based tumor content versus manual estimation in DFCI cohort
eFigure 3. Range of TIL density in different sample types.
eFigure 4. Correlation between TIL levels and TMB/PD-L1.
eFigure 5. TIL levels according to KRAS and EGFR mutation status in the discovery cohort.
eFigure 6. Log-rank test cutoff identification
eFigure 7. TIL levels and overall survival to immunotherapy
eFigure 9. TILs and objective response rate (ORR)
eFigure 10. Treatment line-TILs interaction. Progression-free and overall survival in treatment subgroups of the combined cohorts.
eFigure 11. Combined TILs/PD-L1, TMB/PD-L1 and immunotherapy outcome
eFigure 12. TILs and immunotherapy outcome based on PD-L1 stratification
eFigure 13. Quantification of TILs by machine-learning (ML) methods
eTable 1. Patient characteristics according to TIL levels in the discovery and validation cohorts
eTable 2. Machine-learning derived quantitative detail of the cell subsets from the histological H&E images in the entire cohort, including both the discovery and validation cohorts (n = 685)
eTable 3. Clinicopathologic variables, including TILs, in association with PFS and OS to ICIs in A) discovery and B) validation cohorts (Univariate analyses, Log-rank test, unadjusted Cox proportional hazard ratios)
eTable 4. Comparison of different single and multi-assays sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for responders versus non-responders after ICI treatment in overall discovery cohort and PD-L1 subsets. 95% CI in parentheses