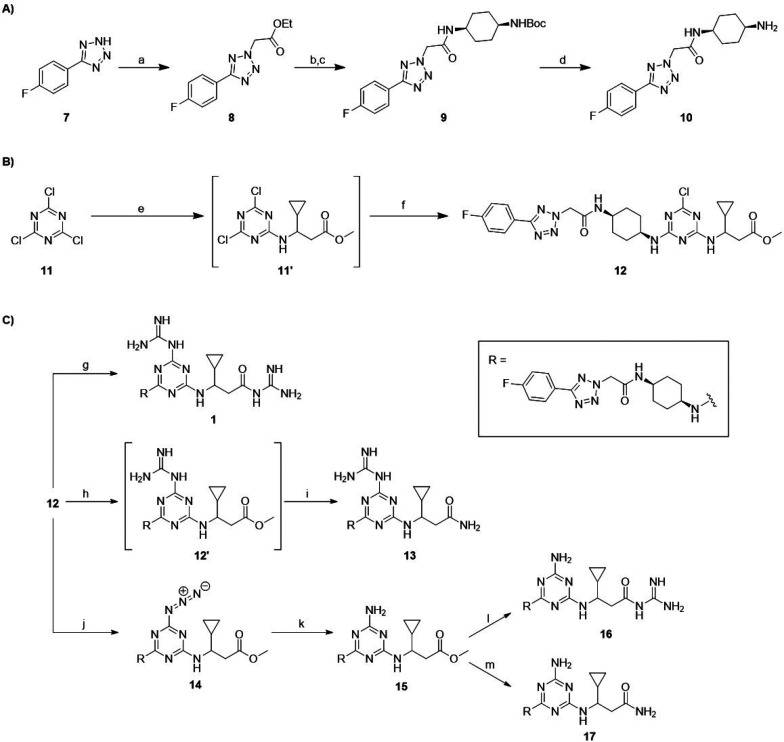
Scheme 1. (A) Synthesis of Building Block 10, (B) Synthesis of Scaffold 12, and (C) Synthesis of MRL-494 (1) and Analogues 13, 16, and 17.
Reagents and conditions for (A): (a) bromoethyl acetate, NaOEt, EtOH, 70 °C, 18 h; (b) 1 M NaOH, THF, rt, 18 h (72% over two steps); (c) 1-N-Boc-cis-1,4-cyclohexanediamine, NEt3, HBTU, DCM, rt, 18 h (90%); (d) TFA, DCM, rt, 3 h (quant).
Reagents and conditions for (B): (e) (±)-methyl 3-amino-3-cyclopropylpropanoate·HCl, DIPEA, ACN, −10 °C to rt, 2 h; (f) 10, DIPEA, ACN, rt, 18 h (55% over two steps).
Reagents and conditions for (C): (g) guanidine·HCl, NaH, DABCO, DMF, rt, 18 h (54%); (h) guanidine·HCl, NaH, DABCO, DMF, rt, 18 h; (i) 7 M NH3 in MeOH, DABCO, 65 °C, 96 h (35% over two steps); (j) NaN3, DMF, 80 °C, 18 h (51%); (k) PPh3, pyridine, H2O, 55 °C, 18 h (50%); (l) guanidine·HCl, NaH, DABCO, DMF, rt, 72 h (51%); (m) 7 M NH3 in MeOH, DABCO, 65 °C, 2 wks (41%).