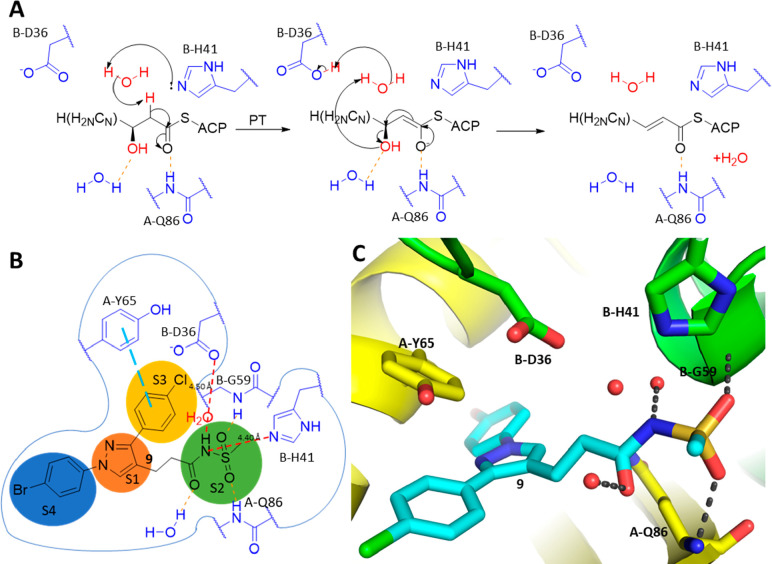
Figure 4.
Acyl sulfonamide inhibitor 9 binds to the active site of Mtb HadAB, mimicking the high energy oxyanion intermediate of the fatty acid-ACP dehydratase reaction. (A) Generalized fatty acid-ACP dehydratase mechanism facilitated in the binding site of the HadAB complex. Substrate binding is facilitated by hydrogen bonds to the backbone amide of A-Q86 and a fixed water. (B) 2D schematic of the HadAB binding site. Here four subsites are defined as S1, S2, S3, and S4, respectively, each accommodating a specific group from the bound ligand. The acyl sulfonamide moiety binding to the S2 subsite mimics the H-bonding pattern of the high energy oxyanion intermediate of the fatty acid-ACP dehydratase reaction, with the enolizable sulfonamide N equidistant (4.4–4.5 Å; red dashed lines) from the centers of the basic N of B-H41 and an O of B-D36, ideally located for a water-mediated deprotonation and proton transfer. The S3 subsite leads to an opening of the HadAB heterodimeric complex toward solvent. This channel features a prominent aromatic residue B-Y65, forming a π-stacking interaction with the chlorophenyl group. S1 is a nonspecific subsite that fits several heterocyclic cores including pyrazole. S4 is a hydrophobic cavity bound by nonaromatic lipophilic residues like B-L91 and B-I60. It binds large hydrophobic groups like the bromophenyl moiety. (C) 3D illustration of the binding mode of 9 interacting with the HadAB complex with the HadA subunit depicted in yellow and the HadB subunit depicted in green. Ligand 9 is depicted in cyan and the H-bonds are depicted in gray. This view focuses on the H-bond interactions between the acyl sulfonamide moiety of 9 and the backbone amides of A-Q86, B-G59, and a fixed water. A catalytic water is trapped between the catalytic B-D36 and B-H41 residues, bridging them via H-bonds. A-Y65 and the chlorophenyl moiety are also shown to be lined up with π-stacking interactions, while the bromophenyl moiety is shown to extend into a lipophilic pocket toward the back of this view (PDB ID: 7SVT).