Summary
Polycomb dictates developmental programs in higher eukaryotes, including flowering plants. A phytohormone, Abscisic acid (ABA), plays a pivotal role in seed and seedling development and mediates responses to multiple environmental stresses, such as salinity and drought.
In this study, we show that ABA affects the Polycomb Repressive Complex 2 (PRC2)-mediated Histone H3 Lys 27 trimethylation (H3K27me3) through VIN3-LIKE1/VERNALIZATION 5 (VIL1/VRN5) to fine-tune the timely repression of ABSCISIC ACID INSENSITIVE 3 (ABI3) and ABSCISIC ACID INSENSITIVE 4 (ABI4) in Arabidopsis thaliana.
vil1 mutants exhibit hypersensitivity to ABA during early seed germination and show enhanced drought tolerance.
Our study revealed that the ABA signaling pathway utilizes a facultative component of the chromatin remodeling complex to demarcate the level of expression of ABA-responsive genes.
Keywords: Arabidopsis, ABA, germination, drought tolerance, VIL1, PRC2
Introduction
Abscisic acid (ABA) is a phytohormone that plays essential roles in seed dormancy, seed germination, seedling growth, stomata closure, water usage, and stress responses (Cutler et al., 2010; Chen, K et al., 2020; Zhang et al., 2020). ABA accumulates in the developing embryo but declines rapidly upon imbibition, which precedes seed germination and early seedling growth (Gubler et al., 2005; Weitbrecht et al., 2011). Exogenous ABA treatment inhibits seed germination and early seedling growth, and several ABA-insensitive (ABI) genes have been identified based on the lack of response to the ABA treatment (Finkelstein et al., 2002). Among ABI genes, ABI3, ABI4, and ABI5 encode transcription factors containing B3, APETALA2-like, and basic leucine zipper (bZIP) domains for DNA bindings, respectively (Giraudat et al., 1992; Finkelstein et al., 1998; Finkelstein & Lynch, 2000). ABI1 and ABI2 are involved in ABA signaling and encode protein phosphatase 2Cs (PP2Cs) (Cutler et al., 2010; Chen, K et al., 2020). PYR/PYL/RCAR family of ABA receptors antagonize PP2Cs, including ABI1 and ABI2, upon the ABA binding, and this, in turn, results in the accumulation of phosphorylated forms of SNF1-related protein kinases (SnRK2s) (Fujii et al., 2009; Ma et al., 2009; Park et al., 2009). Activated SnRK2s phosphorylate several transcription factors to trigger a cascade of transcriptional regulation (Cutler et al., 2010; Chen, K et al., 2020). For example, RAV (Related to ABI3/VP1)-class transcription factor RAV1 is phosphorylated by SnRK2s and regulates expressions of early ABA-responsive transcription factors, including ABI3, ABI4, and ABI5 (Feng et al., 2014). ABI3, ABI4, and ABI5 transcripts accumulate in developing embryos but rapidly decrease upon germination and during early seedling development, indicating that dynamic transcriptional regulatory network functions to control the expression of ABI3, ABI4, and ABI5 during seed and early seedling development (Jia et al., 2014; Chandrasekaran et al., 2020). In addition, these transcription factors are induced upon the ABA treatment by triggering the ABA signaling pathway to mediate downstream transcriptional cascades (Jia et al., 2014; Chandrasekaran et al., 2020).
Polycomb Repressive Complex 2 (PRC2) is an evolutionarily conserved chromatin-modifying enzyme complex that mediates Histone H3 Lys 27 trimethylation (H3K27me3) at developmentally controlled loci in eukaryotes (Mozgova & Hennig, 2015; Yu et al., 2019). PRC2 consists of four core subunits, including Enhancer of Zeste (E(z)), Extra Sex Combs (ESC), WD40-containing protein (Nurf55), and Suppressor of Zeste 12 (Su(z)12) in a stoichiometric ratio of 1:1:1:1 (Ciferri et al., 2012). In Arabidopsis, multiple genes encode four core subunits with some functional redundancies (Mozgova & Hennig, 2015). Three homologous genes for the E(z) methyltransferases are MEDEA (MEA), CURLY LEAF (CLF), and SWINGER (SWN), and a single homologous gene for ESC is FERTILIZATION INDEPENDENT ENDOSPERM (FIE) (Mozgova & Hennig, 2015). Five Nurf55-like genes, MULTICOPY SUPPRESSOR OF IRA1-5 (MSI1-5), and three Su(z)12-like genes, FERTILIZATION INDEPENDENT SEED 2 (FIS2), EMBRYONIC FLOWER2 (EMF2), and VERNALIZATION2 (VRN2), exist in the Arabidopsis genome (Mozgova & Hennig, 2015). Three distinct PRC2 complexes function in various aspects of developmental transitions in Arabidopsis. The FIS2 complex uses MEA as a sole E(z) methyltransferase and includes FIS2, FIE, and MSI1 (Baroux et al., 2006; Hennig & Derkacheva, 2009). The FIS2 complex functions only in the female gametophyte and endosperm but not in the sporophyte (Hennig & Derkacheva, 2009). In sporophyte, two PRC2 complexes, the EMF2 complex (EMF2, FIE, CLF or SWN, and MSIs) and the VRN2 complex (VRN2, FIE, CLF or SWN, and MSIs), function by utilizing two E(z) homologs, CLF and SWN, redundantly (Chanvivattana et al., 2004; De Lucia et al., 2008; Hennig & Derkacheva, 2009; Mozgova & Hennig, 2015). clf swn double mutants exhibit multiple developmental defects including developmental arrest at seedling stage, illustrating the pivotal function of PRC2 complexes in plant development (Chanvivattana et al., 2004; Farrona et al., 2011; Shu et al., 2019). The EMF2 complex is involved in developmental transitions, and the VRN2 complex is known to repress FLOWERING LOCUS C (FLC) in response to winter cold (Gendall et al., 2001; De Lucia et al., 2008; Kim et al., 2012). In mammals and Drosophila, the core of PRC2 is also associated with a number of facultative subunits which facilitate the PRC2 activity, providing a regulatory flexibility in regulating gene expression (Mozgova & Hennig, 2015; Yu et al., 2019). Similarly, genetic and biochemical studies revealed that the VRN2 complex is associated with the PHD-finger containing the VERNALIZATION INSENSITIVE 3 (VIN3) family proteins, including VIN3, VIN3-LIKE 1 (VIL1), and VIL2 (De Lucia et al., 2008). The VIN3 family proteins are necessary for the repression of FLC by the VRN2 complex (De Lucia et al., 2008; Kim & Sung, 2013).
Although PRC2 plays roles in many aspects of plant development, its involvement in seed germination has not been studied. Seed germination is controlled in part by ABA signaling pathway that determined the balance between seed dormancy and germination (Jia et al., 2014). The ABA signaling pathway eventually elicits the cascade of transcriptional regulations, and several key transcription factors have been identified (Chen, K et al., 2020; Zhang et al., 2020). Although the prominent roles of the DNA-binding transcription factors in ABA signaling pathway have been extensively studied (Chen, K et al., 2020), chromatin-modifying complexes are also expected to be involved in transcriptional regulation of the ABA signaling pathway (Peirats-Llobet et al., 2016; Bulgakov et al., 2019; Lee & Seo, 2019; Liu et al., 2019). However, defects in many chromatin-remodeling complexes also compromise multiple developmental processes which make it difficult to address biological significance of the roles of chromatin-remodeling complexes in the ABA signaling. Here, we report that the vil1 mutant is hypersensitive to the ABA treatment and exhibits enhanced drought tolerance. Our study shows that VIL1-PRC2 is necessary to properly repress ABA-signaling transcription factors, including ABI3 and ABI4. We identified the molecular regulatory mechanism by which VIL1 limits the induction of ABA-responsive genes to coordinately regulate ABA-mediated transcriptional changes that lead to proper early seedling development and drought response.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana (L.) accession Columbia (Col-0), vil1 (SALK_136506), vil1-2 (SALK_140132), clf (SALK_106381), swn (SALK_050195), abi3 (SALK_138922), abi4 (CS8104) and abi5 (SALK_013163) lines were obtained from Arabidopsis Biological Resource Center (Columbus, OH). To generate complement lines pVIL1:VIL1-myc/vil1 and pVIL1:VIL1-flag/vil1, the VIL1 genomic DNA was first cloned into the pENTR_dTOPO vector and further transferred to pGWB16 or pEarleyGate 302 vectors, respectively. vil1 abi3_cri was generated by CRISPR/Cas9 method (Xing et al., 2014; Wang et al., 2015; Liu et al., 2017). The constructs above were transformed into Agrobacterium tumefaciens cells (GV3101) and then stably transformed into Arabidopsis using the floral dip method (Clough & Bent, 1998). The complementation lines of pCLF:CLF-GFP/clf29 (gCLF-GFP) and pSWN:SWN-GFP/swn-4 (gSWN-GFP) were previously described (Shu et al., 2019). Higher-order mutants were generated by genetic crossing. Plants were grown in controlled environmental chambers with cool white fluorescent lights and maintained at 22°C. The photoperiodic cycle was 16 h light/8 h dark (long day, LD) and 8 h light/16 h dark (short day, SD).
ABA treatment
Seeds collected at the same time were used for ABA treatment assays. For germination assays, surface-sterilized seeds were plated on half-strength Murashige & Skoog medium (½MS medium) supplemented with or without the indicated concentration of ABA and then stratified at 4°C in darkness for 3 days. The germination rates (green cotyledon) were counted at the indicated time points. For root growth assays, seeds were germinated on ½MS medium for 3 days, followed by transfer to ½MS medium with or without 10 μM ABA. Plates were incubated vertically for an additional 7 days before measuring primary root length by Image J. For long-term ABA treatment for gene expression and ChIP analysis, seeds were spread on a filter paper, which plated on ½MS medium supplemented with or without 0.5 μM ABA and then stratified at 4°C in darkness for 3 days followed by growth in long-day growth chambers for another 3 days. For short-term ABA treatment for gene expression and ChIP analysis, seeds were spread on a filter paper, which plated on ½MS medium and then stratified at 4°C in darkness for 3 days followed by growth in long-day growth chambers for another 3 days. Filter papers with germinated seedlings were moved into liquid ½ MS medium with or without 50 μM ABA for 4 h.
Drought stress and dehydration treatment
For the drought tolerance analysis, 5-day-old seedlings grown on ½ MS medium were transferred to thoroughly watered soil: turface (3:1) and grown in 12h light /12 dark condition. To avoid positional bias, different genotypes were grown at various sites of the pot. After 20 days of growth without water, watering was resumed, and phenotypes were recorded 3 days after watering. For dehydration treatment, 7-day-old seedlings grown on ½ MS medium were exposed to the air by removing the petri dish lid. After 5 days, seedlings were rehydrated. Survival rates were counted, and pictures were taken 1 day after rehydration. Seedlings that have more than two green leaves were counted as surviving.
Water loss Experiments
For water loss rate measurement, rosette leaves were detached from 3-week-old plants grown in long-day condition or 5-week-old plants grown in short-day condition, weighed immediately on weighting paper, and then placed on the laboratory bench. The weight losses of the samples were measured at designated time points (as indicated in Fig. 7). The proportion of water loss rate was calculated based on the initial fresh weight of the samples.
Fig. 7.
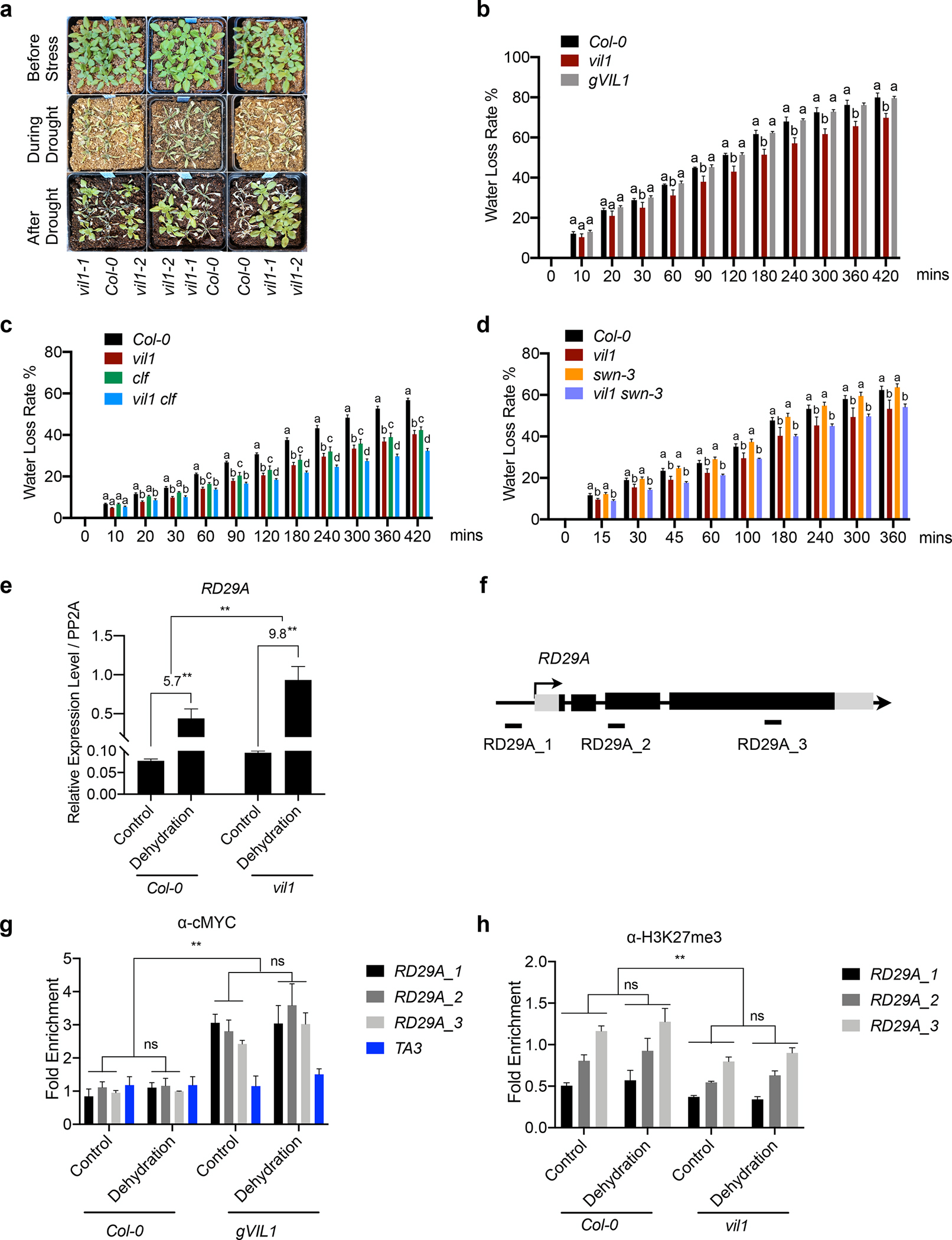
Enhanced drought tolerance of vil1 mutants in Arabidopsis. (a) Plant phenotypes for indicated genotypes before and after drought stresses. The phenotype of plants before drought (top), during (middle), and after re-watering (bottom). (b to d) Detached leaf water loss assays. Plants of Col-0, vil1, and gVIL1 are grown in long days (b). Plants of Col-0, vil1, clf, and vil1 clf are grown in short days (c). Plants of Col-0, vil1, swn-3, and vil1 swn-3 are grown in long days (d). Leaves with similar developmental stages were detached and weighted at the indicated time. Water loss represents the proportion of total weight loss compared with initial weight. Error bars: ±s. d. (n = 3). One-way ANOVA Tukey’s multiple comparison test was conducted; letters indicate P < 0.05 of distinct groups. (e) RT-qPCR analysis of RD29A expression under dehydration stress in Col-0 and vil1. Transcript levels were normalized to PP2A and error bars: ±s. d. (n = 3). The numbers (5.7 and 9.8) indicate fold changes between control and dehydration conditions in each genotype. (f) Schematic diagram showing the genome regions of RD29A. Exons are represented by black boxes, while black lines between exons represent introns. Black arrow with a vertical line indicates the transcription start site and the direction of arrow indicates the orientation of transcription. DNA fragments amplified in ChIP assays are labeled beneath the genomic regions. (g) Analysis of VIL1 binding to RD29A genomic regions with or without dehydration treatment for 1 hour. Enriched values were normalized with the level of input DNA and relative fold enrichment over Col-0 are presented. Error bars: ± s. d. (n = 3). (h) ChIP-qPCR analysis of H3K27me3 levels at RD29A in the Col-0 and vil1-1 seedlings with or without dehydration treatment for 1 hour. Each examined region was normalized to TA3 and error bars: ± s. d. (n = 3). **P < 0.01 and ns indicate no significant differences between distinct groups. Significant difference using Student’s t-test.
mRNA expression analysis
Total RNA was prepared using PureLink™ Plant RNA Reagent (Invitrogen). For reverse-transcription followed by quantitative PCR (RT-qPCR), 1 μg total RNAs were treated with DNase I (Invitrogen) before reverse transcription, and then the first-strand cDNA was synthesized by M-MLV (Invitrogen). RT-qPCR was performed using ViiA 7 Real-Time PCR System (Applied Biosystems) and AzuraQuant™ Green Fast qPCR Mix (Azura Genomics). Arabidopsis constitutively expressed PP2A (AT1g69960) was used as an internal control for normalization. The primers used for RT-qPCR are listed in Table S1.
Protein Extraction and western blot
For protein extraction, seedlings were frozen in liquid nitrogen and homogenized with urea-denaturing buffer (100 mM NaH2PO4, 10 mM Tris-HCl (pH 8.0), 8M Urea, 1 mM PMSF, Protease inhibitor cocktails). The debris was removed by centrifugation at 12,000 g at 4°C for 10 min. Extracted proteins were denatured by boiling at 100°C for 10 min with 1X SDS sample buffer. Western blot was performed to check VIL1-myc protein levels using anti-myc antibody (Santa Cruz, c-myc (9E10) X antibody, sc-40X, 1:5,000 dilution). Tubulin levels were detected by anti-α-Tubulin antibody (T5168, 1:10,000 dilution). Ponceau S staining was performed to minimize any discrepancies in protein amount.
RNA-Seq analysis
The total RNA was extracted from 1-day-old seedlings using PureLink™ Plant RNA Reagent (Invitrogen). For each sample, three independent replicates were used. Genomic Sequencing and Analysis Facility, the University of Texas at Austin, performed the library preparation and sequencing. Briefly, a total amount of 1 μg RNA per sample was used for 3’-TagSeq library preparations. Prepared libraries were sequenced by NovaSeq 6000 System (Illumina). The reads were mapped to the TAIR 10 Arabidopsis genome using Bowtie 2. Differentially expressed genes were identified by using DESeq 2 with default parameters. Genes with at least a 1.5-fold change in expression and P < 0.05 between mutant and WT (Col-0) were considered differentially expressed genes (DEGs). The gene ontology of DEGs in vil1 mutant was performed by the AgriGO v2.0 with default parameters (Tian et al., 2017). The Heat map to show the enriched gene ontology categories was performed by TBtools (Chen, C et al., 2020).
Chromatin immunoprecipitation (ChIP)
ChIP experiments were performed as previously reported (Zong et al., 2021). Briefly, 1 g seedlings were used for nuclei isolation. Chromatin was sheared by using a Bioruptor® to an average size of about 500 bp. Sheared chromatin was further diluted in ChIP dilution buffer and incubated with 4 μg (Santa Cruz, c-myc (9E10) X antibody, sc-40X), H3K27me3 (07–449; Millipore Sigma) or GFP (ab290; Abcam) antibodies overnight at 4°C. Antibody was further captured by Dynabeads Protein G (Thermo Fisher Scientific) and then washed by low salt, high salt, LiCl, and TE buffers. The DNA-protein complex was eluted, and reverse cross-linked at 65°C overnight. DNA was purified by using QIAquick® PCR Purification Kit (Qiagen) and was used for qPCR analysis (Primers listed in Table S1).
Statistical analysis
Two-tailed Student’s t-test and One-way ANOVA followed by Tukey HSD test for multiple comparisons were conducted using Prism 8.
Results
VIL1 negatively regulates the ABA response
Although VIL1 is best known as a component of PRC2 that mediates H3K27me3 at the FLC locus upon vernalization (Sung et al., 2006; Greb et al., 2007; De Lucia et al., 2008), VIL1 also appears to function in other developmental processes, including photoperiodic flowering (Sung et al., 2006) and light signaling (Kim et al., 2021). To elucidate the roles of VIL1 in early developmental processes, we performed transcriptome analysis using 1-day-old seedlings of wild type and vil1 mutants (Fig. S1a; Table S1). Gene ontology (GO) analysis of differentially expressed genes (DEGs) in vil1 mutants showed that GO terms of stress responses, hormone signaling, and seed germination processes are significantly enriched in up-regulated DEGs but not in down-regulated DEGs in vil1 mutants (Fig. S1b). Interestingly, terms related to “in response to abscisic acid (ABA)” are among significant up-regulated DEGs in vil1 mutants. VIL1 is a transcriptional repressor as a component of PRC2 (Sung et al., 2006; Greb et al., 2007; De Lucia et al., 2008). Therefore, de-repression of ABA-related genes in vil1 mutants implies that VIL1 plays role in ABA-related responses through gene repression.
To investigate whether VIL1 is involved in the ABA responses, we first examined the germination response in vil1 mutants. Interestingly, the germination rate of the vil1 mutants was significantly decreased after the ABA treatment when compared to the wild-type (WT, Col-0) and the complemented line (gVIL1) (Fig. 1a,b). The ABA hypersensitive phenotype of vil1 mutants was further confirmed by using another allele (vil1-2) and an additional complemented line (gVIL1-FLAG) (Fig. S2). Moreover, the growth of the primary root of vil1 mutants was also inhibited by the ABA treatment to a greater extent than WT root (Fig. 1c,d). Thus, these results indicate that VIL1 negatively regulates the ABA signaling in Arabidopsis.
Fig. 1.
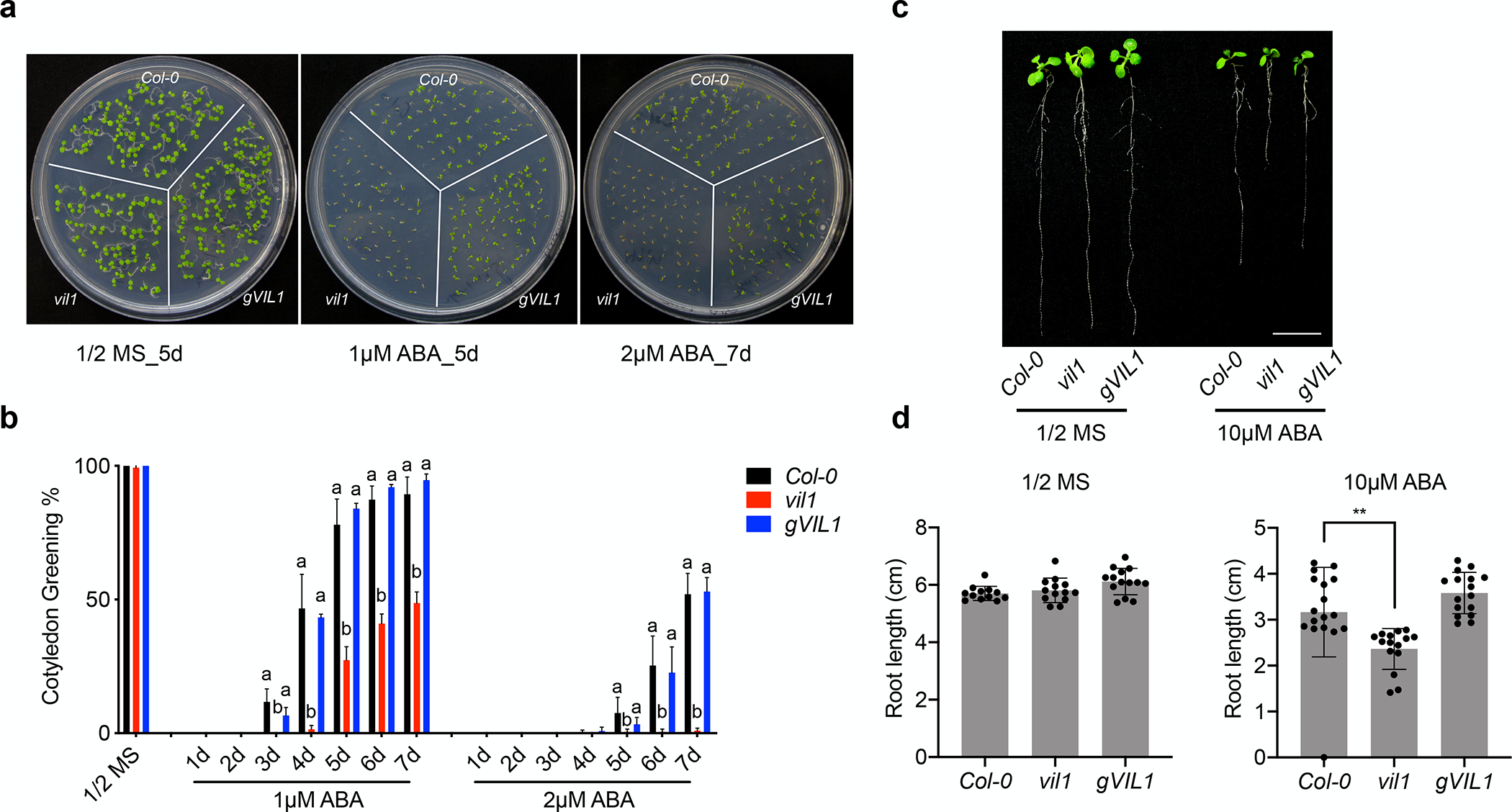
vil1 mutant shows increased ABA sensitivity in Arabidopsis. (a) Germination phenotypes of WT, vil1 mutants, and complement lines (gVIL1-cMYC, gVIL1) in the presence of 1 μM or 2 μM ABA. (b) The percentage of germinated embryos that developed green cotyledons in the presence of 1 μM or 2 μM ABA in the Col-0, vil1, and gVIL1. Values are mean ± s. d. (n = 3; each set includes more than 60 seeds). One-way ANOVA Tukey’s multiple comparison test was conducted; letters indicate P < 0.05 of distinct groups. (c) Root phenotype of Col-0, vil1, and gVIL1 plants grown under 1/2 MS medium or 1/2 MS medium containing 10 μM ABA for 7 days. White scale bar = 1 cm. (d) Primary root length of seedlings treated as described in (c). The black dots indicate individual measurement. Data are presented as the means ± s. d. (n = 15). **P < 0.01, significant difference using Student’s t-test.
VIL1 directly represses ABI3 and ABI4
To understand how VIL1 regulates the ABA responses in Arabidopsis, we further investigated the gene expression profiles in vil1 mutants. A total of 919 DEGs were identified in 1-day-old seedlings of vil1 mutants (Fig. S1a; Table S2). Three ABA signaling transcription factors, ABI3, ABI4, and ABI5, were among the up-regulated DEGs in vil1 mutants. These three transcription factors are rather quickly repressed during early seed germination process and constitute a crucial transcription regulatory hub in ABA signaling (Lopez-Molina et al., 2001; Perruc et al., 2007; Chandrasekaran et al., 2020). To test whether VIL1 was required for the repression of ABI3, ABI4, and ABI5 during the seed germination, we first investigated the expression levels of ABI3, ABI4, and ABI5 in the vil1 mutant at different germination stages. Interestingly, we found that ABI3, ABI4, and ABI5 are increased in the vil1 mutant only during the early germination stages (1 to 5 days after germination (DAG)), but not in dry seeds or at later germination stage (7 DAG) (Fig. S3), suggesting that VIL1 is necessary for establishing the repression of these transcription factor genes during early stages of germination.
ABA treatment increases the expression of ABI3, ABI4, and ABI5 in WT (Perruc et al., 2007; Chandrasekaran et al., 2020). Interestingly, the expression levels of ABI3, ABI4, and ABI5 are even higher in vil1 mutants compared to WT (Fig. 2a), indicating that VIL1 limits the induction of ABI3, ABI4, and ABI5 upon the ABA treatment. To explore whether VIL1 directly binds to ABI3, ABI4, and ABI5 genomic regions, we employed chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR) assays. Indeed, we observed the enrichment of VIL1 at ABI3 and ABI4 genomic regions, but not at ABI5 locus, at 3 DAG (Fig. 2b–d). Interestingly, the levels of VIL1 enrichment at ABI3 and ABI4 loci significantly decreased at the gene body regions of ABI3 and ABI4 when treated with ABA (Fig. 2b–d), indicating that the removal of VIL1 from ABI3 and ABI4 chromatin occurs in response to ABA treatment, concurrent with the de-repression of ABI3 and ABI4.
Fig. 2.
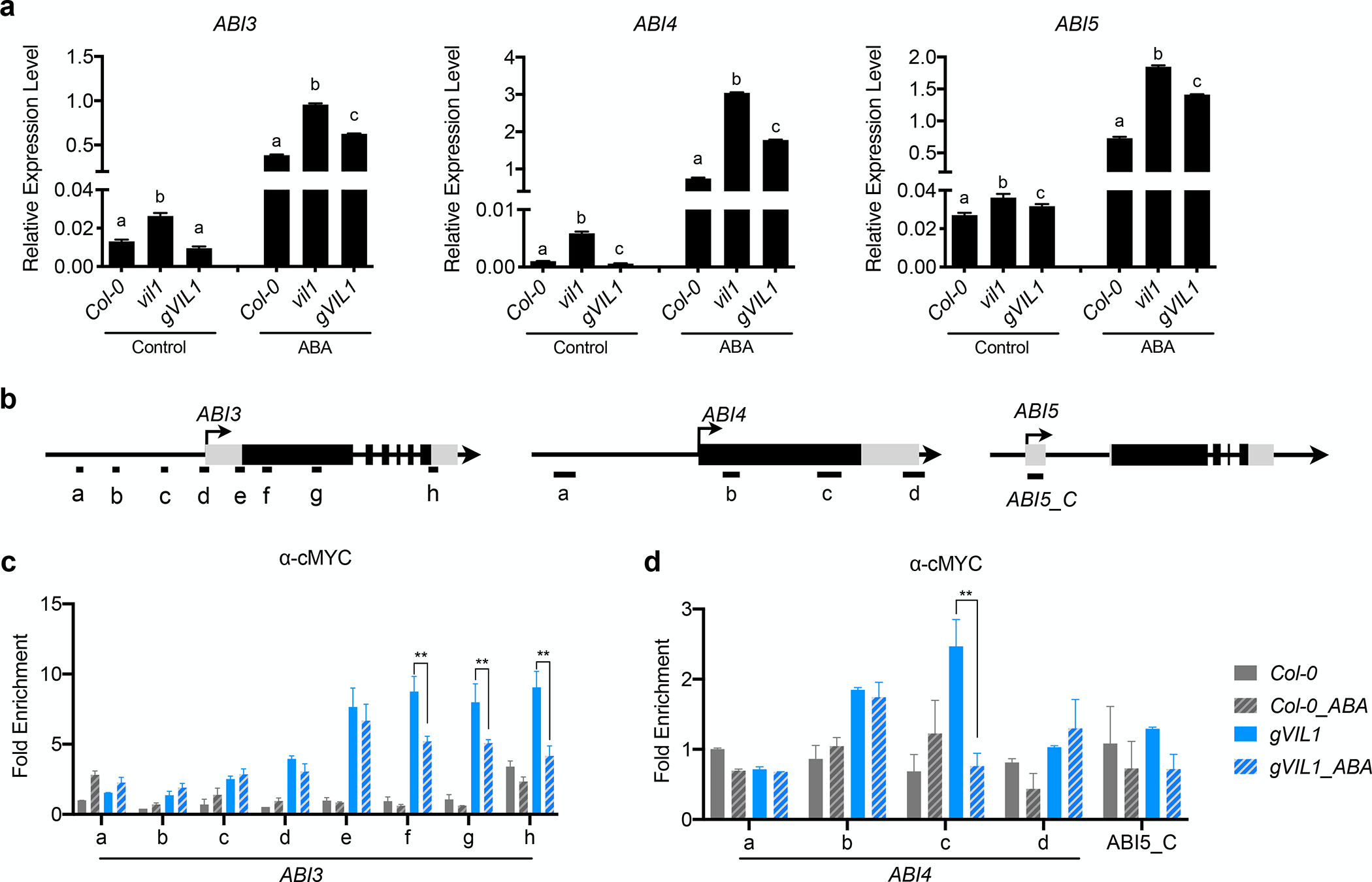
VIL1 directly and negatively regulates the expression of ABI3 and ABI4 in Arabidopsis. (a) Relative expression of ABI3, ABI4, and ABI5 in Col-0, vil1, and gVIL1 seedlings germinated with or without ABA. Stratified Col-0, vil1, and gVIL1 seeds were germinated with (ABA) or without (Control) 0.5 μM ABA for 3 days. Transcript levels were normalized to the level of PP2A. Error bars: ± s. d. (n = 3). One-way ANOVA Tukey’s multiple comparison test was conducted; letters indicate P < 0.05 of distinct groups. (b) Schematic diagram showing the genome regions of ABI3, ABI4, and ABI5. Exons are represented by black boxes, while black lines between exons represent introns. Black arrows with vertical lines indicate transcription start sites and direction of arrows indicate the orientation of transcription. DNA fragments amplified in ChIP assays are labeled beneath the genomic regions. (c and d) Analysis of the VIL1 binding to ABI3, ABI4, and ABI5 genomic regions with or without 0.5 μM ABA for 3 days. Enriched values were normalized to the level of input DNA, and the relative fold enrichment over Col-0 is presented. Error bars: ± s. d. (n = 3). (c,d) **P < 0.01. Significant difference using Student’s t-test.
VIL1 is necessary for the H3K27me3-mediated repression of ABI3 and ABI4
Because VIL1 is known to promote PRC2 function, the H3K27 methylation, we also measured the level of H3K27me3 at ABI3, ABI4, and ABI5 loci (Fig. 3a,b). Consistent with the direct association of VIL1 with ABI3 and ABI4 chromatin, the levels of H3K27me3 are significantly lower at ABI3 and ABI4 loci in vil1 mutants (Fig. 3a,b). Furthermore, ABA treatment reduces the level of H3K27me3 at ABI3 and ABI4 loci (Fig. 3a,b), indicating that the reduction in VIL1 enrichment by ABA results in the decrease in the levels of H3K27me3 at ABI3 and ABI4. On the other hand, no significant H3K27me3 enrichment was observed at the ABI5 locus regardless of the ABA treatment (Fig. 3b), indicating that ABI5 is not under the control of VIL1-mediated H3K27me3 (Fig. 2d, 3b).
Fig. 3.
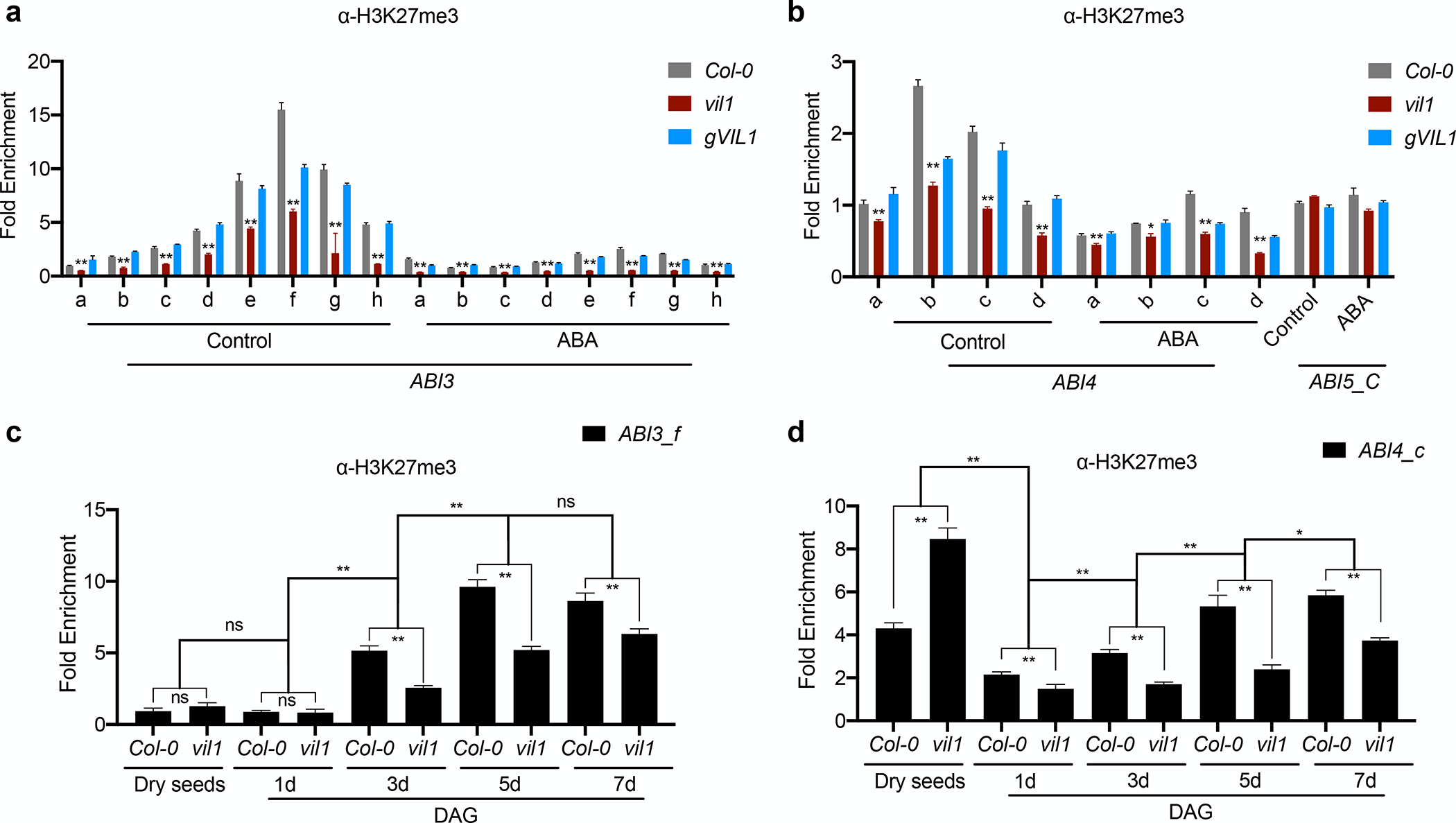
VIL1 is necessary for the H3K27me3 enrichment at ABI3 and ABI4 loci in Arabidopsis. (a and b). ChIP-qPCR analysis of H3K27me3 at ABI3, ABI4, and ABI5 loci in the Col-0, vil1-1, and gVIL1 seedlings germinated with (ABA) or without (Control) 0.5 μM ABA for 3 days. Each examined region was normalized to Input and error bars: ± s. d. (n = 3). *P < 0.05, **P <0.01, Significant difference using Student’s t-test. (c and d) ChIP-qPCR analysis of H3K27me3 levels at ABI3 and ABI4 at different germination stages of the Col-0 and vil1-1. For dry seeds of Col-0 and vil1, seeds were sterilized and imbibed in water for 30 mins and then harvested for ChIP analysis. For germinated seeds, Col-0 and vil1 seeds were first sterilized and stratified at 4°C for 3 days and then germinated at 22°C with 16h light /8h dark condition. Each examined region (depicted in Fig. 2b) was normalized to TA3 and error bars: ± s. d. (n = 3). *P < 0.05, **P < 0.01, ns no significant differences between distinct groups. Significant difference using Student’s t-test.
Transcriptional repression is triggered at ABI3, ABI4, and ABI5 loci during early germination (Lopez-Molina et al., 2001; Perruc et al., 2007; Chandrasekaran et al., 2020). We observed that the levels of H3K27me3 increase rapidly during early germination at ABI3 and ABI4 chromatin but not at ABI5 chromatin (Fig. 3c,d, Fig. S4). Furthermore, the increases in the level of H3K27me3 at ABI3 and ABI4 are compromised in vil1 mutants (Fig. 3c,d). It is known that the repression of ABI3 and ABI4 after germination is achieved by the various combinations of their transcriptional activators and repressors (Parcy et al., 1997; To et al., 2006; Feng et al., 2014). The reduced levels of H3K27me3 at ABI3 and ABI4 in vil1 mutants correlate with the de-repression of ABI3 and ABI4 at the early stages of germination, but both ABI3 and ABI4 are eventually repressed both in WT and vil1 mutants at later stage of gemination (Fig. S3), indicating that the VIL1-mediated H3K27me3 is necessary for the rapid repression of ABI3 and ABI4 at the early stage of germination. Taken together, our results show that VIL1 directly associates with ABI3 and ABI4 chromatin to repress their expressions by modulating H3K27me3 levels during early seed germination.
ABA-responsive activation of ABI3 and ABI4 includes the removal of VIL1 to reduce H3K27me3
We observed that the ABA-triggered induction of ABI3 and ABI4 accompanies the reduced enrichment of VIL1 and H3K27me3 at ABI3 and ABI4 loci (Fig. 2c,d, Fig. 3a,b). Significant induction of ABI3 and ABI4 transcripts is observed with 4 hours of ABA treatment (Fig. 4a–c). Interestingly, reductions in the enrichment of VIL1 at these loci also occur rapidly as short as 4 hours of the ABA treatment (Fig. 4d). Consistent with the rapid reduction in the enrichment of VIL1, the levels of H3K27me3 at both ABI3 and ABI4 decrease by the 4-hour ABA treatment (Fig. 4e). However, the expression of VIL1 is not affected by the ABA treatment both in the levels of mRNA and protein (Fig. S5). Therefore, ABA affects the VIL1 enrichment at ABI3 and ABI4 loci.
Fig. 4.
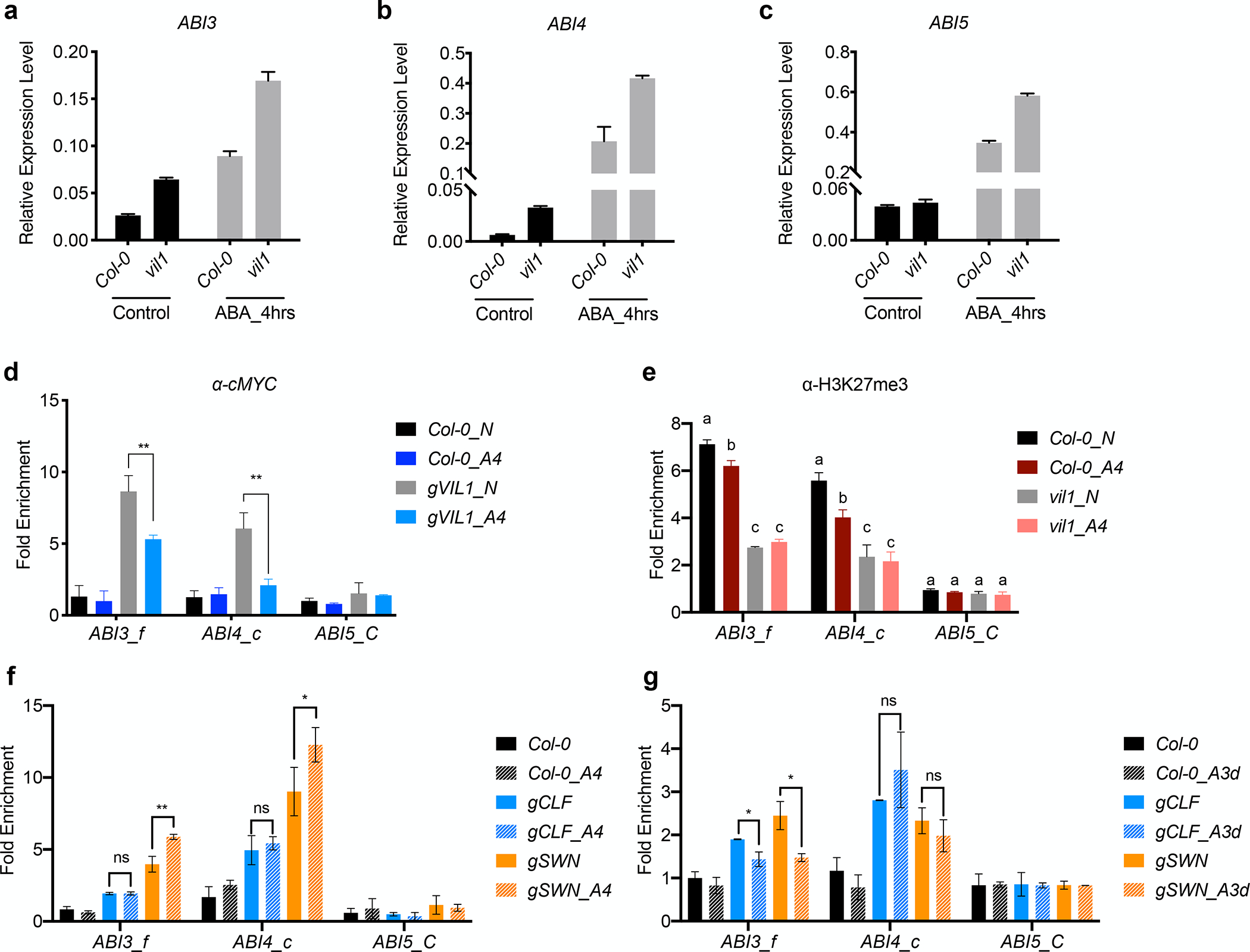
ABA inhibits the H3K27me3 at ABI3 and ABI4 loci in Arabidopsis. (a-c) Relative expression of ABI3 (a), ABI4 (b) and ABI5 (c) in Col-0 and vil1 seedlings treated with or without ABA. 3-day-old Col-0 and vil1 seedlings were treated with (ABA_4hrs) or without (Control) 50 μM ABA for 4 hours. Transcript levels were normalized to PP2A and error bars: ±s. d. (n=3). (d) Analysis of VIL1 binding to ABI3 and ABI4 genomic regions in seedlings treated with or without ABA by ChIP-qPCR. 3-day-old seedlings of Col-0 and gVIL1 were treated with or without 50 μM ABA for 4 hours. Enriched values were normalized with the level of input DNA and relative fold enrichment over Col-0 are presented. Error bars: ± s. d. (n = 3). ABI5 was used as a negative control. **P < 0.01, significant difference using Student’s t-test. (e) ChIP-qPCR analysis of H3K27me3 levels at ABI3 and ABI4 in WT and vil1 seedlings treated with or without ABA. Each examined region was normalized to the retrotransposon TA3 and error bars: ± s. d. (n = 3). ABI5 was used as a negative control. One-way ANOVA Tukey’s multiple comparison test was conducted; letters indicate P < 0.05 of distinct groups. (f and g) Analysis of CLF and SWN binding to ABI3 and ABI4 under short-term (f) and long-term (g) ABA treatment by ChIP-qPCR. For short-term ABA treatment (A4), 3-day-old seedlings of gCLF_GFP, gSWN_GFP and control (Col-0) treated with or without 50 μM ABA for 4 hours were used in ChIP assay. For long-term ABA treatment (A3d), Col-0, gCLF_GFP and gSWN_GFP seedlings that germinated with or without 0.5 μM ABA for 3 days were used in ChIP assay. Fragments immunoprecipitated by anti-GFP were quantified by qPCR and normalized to TA3. Relative levels in gCLF_GFP and gSWN_GFP over control are presented and error bars: ± s. d. (n = 3). ABI5 was used as a negative control. *P < 0.05, **P < 0.01, ns no significant differences between distinct groups, significant difference using Student’s t-test.
Given that VIL1 is a facultative component of PRC2 (De Lucia et al., 2008; Derkacheva et al., 2013; Wang et al., 2016), we first investigated whether ABI3 and ABI4 are direct targets of the core components of PRC2. Indeed, a genome-wide occupancy study of CLF and SWN in Arabidopsis indicates that both CLF and SWN bind to ABI3 and ABI4 loci, but not to ABI5 locus (Fig. S6a), which is consistent with the occupancy patterns of VIL1 (Fig. 2c,d). We also confirmed that CLF and SWN are enriched at ABI3 and ABI4 loci, but not at ABI5 locus by ChIP-qPCR (Fig. S6b,c). In addition, H3K27me3 is not detectable at ABI3 and ABI4 in clf swn double mutants (Shu et al., 2019), indicating that CLF and SWN redundantly control the deposition of H3K27me3 at these loci. On the other hand, ABI5 chromatin is not enriched with H3K27me3, consistent with that ABI5 is not enriched with CLF, SWN, and VIL1 (Fig. 2d, S6a).
Because the VIL1 enrichment at the ABI3 and ABI4 locus is rapidly reduced by ABA treatment (Fig. 4d), we tested whether the core components of PRC2 are also rapidly removed from the ABI3 and ABI4 loci upon the ABA treatment (Fig. 4h). We measured changes in enrichments of two E(z) homologs, CLF and SWN at ABI3 and ABI4 loci upon ABA treatment by ChIP-qPCR. Although CLF and SWN occupy both ABI3 and ABI4 chromatin (Fig. S6a–c), the enrichment of CLF and SWN at ABI3, but not at ABI4, slightly decreases under long-term ABA treatment, and not affected at all by the short-term ABA treatment (Fig. 4f,g). Therefore, the enrichment of VIL1 at ABI3 and ABI4 is critical for the PRC2 activity and its rapid removal by ABA is a part of regulatory modules in the ABA-mediated transcriptional response. Taken together, our data collectively show that the dynamic nature of VIL1-mediated H3K27me3 contributes to the regulation of ABA-responsive genes.
VIL1 mediates the PRC2 activity to specifically regulate the ABA responses
We attempted to address the roles of CLF and SWN in VIL1-mediated ABA responses by examining the ABA responses of the corresponding mutants (Fig. S7). Unlike the vil1 mutant, which is hypersensitive to ABA, neither clf nor swn single mutant shows strong ABA hypersensitivity as determined by cotyledon greening (Fig. S7). The clf single mutant is slightly hyposensitive to the ABA treatment, and the vil1 clf double mutant also shows a higher cotyledon-greening rate than the vil1 single mutant (Fig. S7a,b). On the other hand, the swn single mutant exhibits a slight ABA hypersensitive phenotype similar to the vil1 mutant, and the vil1 swn double mutant shows a synergistically enhanced ABA sensitivity (Fig. S7c,d). The clf swn double mutant exhibits aberrant early seedling development (Shu et al., 2019), and therefore we could not address the ABA responses in clf swn double mutants. In clf mutants, both ABI3 and ABI4 are slightly lower than WT at 3 DAG, while the expressions of ABI3 and ABI4 are not significantly changed in swn mutants (Fig. 5a,b). Neither clf nor swn single mutants significantly de-repress the expression of ABI3 and ABI4 to the comparable level observed in the vil1 mutant (Fig. 5a,b), indicating that CLF and SWN redundantly function together with VIL1 to de-repress ABI3 and ABI4 during early seed germination. We also found the H3K27me3 levels at ABI3 and ABI4 chromatin were largely correlated with the expression levels of ABI3 and ABI4 (Fig. 5c,d). Interestingly, the level of ABI3 expression is lower in clf mutants at 3 DAG, consistent with the slightly higher level of H3K27me3 at ABI3 in clf mutants (Fig. 5a). This observation may indicate possible over-compensation by SWN in clf mutants or other ABA-signaling factors under the control of CLF. Our results indicate that VIL1 promotes the function of both CLF and SWN during seed germination to properly repress ABI3 and ABI4 in the absence of ABA.
Fig. 5.
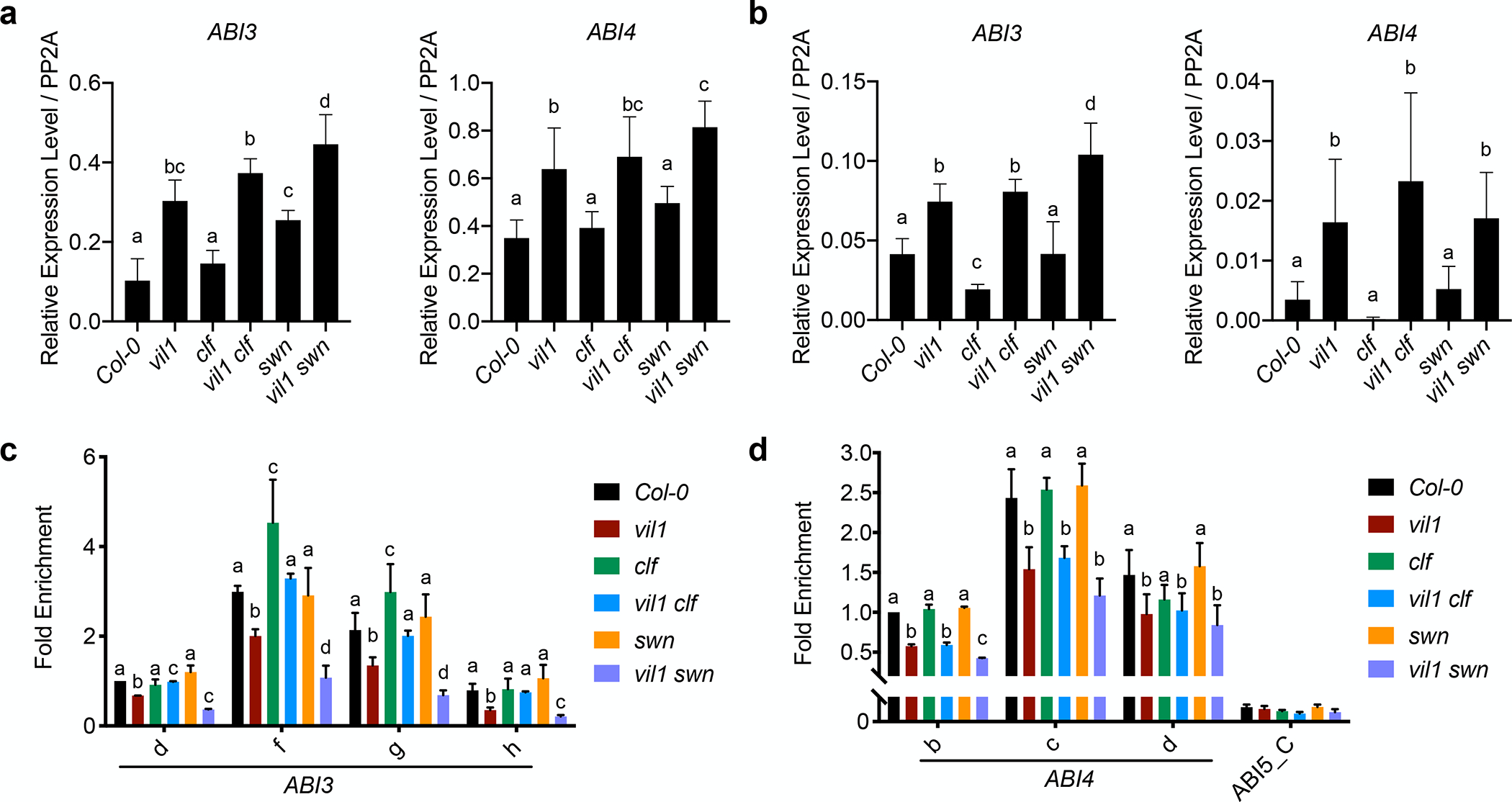
VIL1 promotes the function of both CLF and SWN of PRC2 in Arabidopsis. (a and b) RT-qPCR analysis of ABI3 and ABI4 expression in 1-day (a) and 3-day (b) germinated seeds of the indicated genotypes. Transcript levels were normalized to PP2A and error bars: ± s. d. (n = 3). (c and d) ChIP-qPCR analysis of the H3K27me3 levels at ABI3 (c) and ABI4 (d) loci in indicated genotypes. 3-day-old seedlings of Col-0, vil1, clf, vil1 clf, swn, and vil1 swn are used in ChIP assay. Each examined region was normalized to TA3. Error bars: ± s. d. (n = 3). One-way ANOVA Tukey’s multiple comparison test was conducted; letters indicate P < 0.05 of distinct groups.
VIL1 regulates ABA signaling through ABI3 and ABI4
Our data show that VIL1 is directly associated with ABI3 and ABI4 to repress their expressions during early germination (Fig. 2). VIL1/PRC2-mediated H3K27me3 appears not to control the expression of ABI5 directly. The differential expression of ABI5 observed in vil1 mutants is likely because ABI5 acts downstream of ABI3 and ABI4 (Lopez-Molina et al., 2002; Bossi et al., 2009). To genetically determine whether the increased levels of ABI3, ABI4, and ABI5 caused the ABA hypersensitive phenotype of vil1 mutants, we examined the ABA response of vil1 abi5 and vil1 abi4 double mutants. vil1 abi5 double mutants restore the ABA insensitive phenotype of abi5 single mutant, but vil1 abi5 double mutants rather slowly restored the ABA insensitive phenotype of abi5 at the later stage of germination (Fig. 6a). The ABA hypersensitive effect by the vil1 mutant reverses in vil1 abi4 double mutants and the double mutants show no difference compared to abi4 single mutants after 3 DAG (Fig. 6b), although the ABA insensitive phenotype of abi4 was partially restored in vil1 abi4 double mutants at the early stage of germination (Fig. 6b).
Fig. 6.
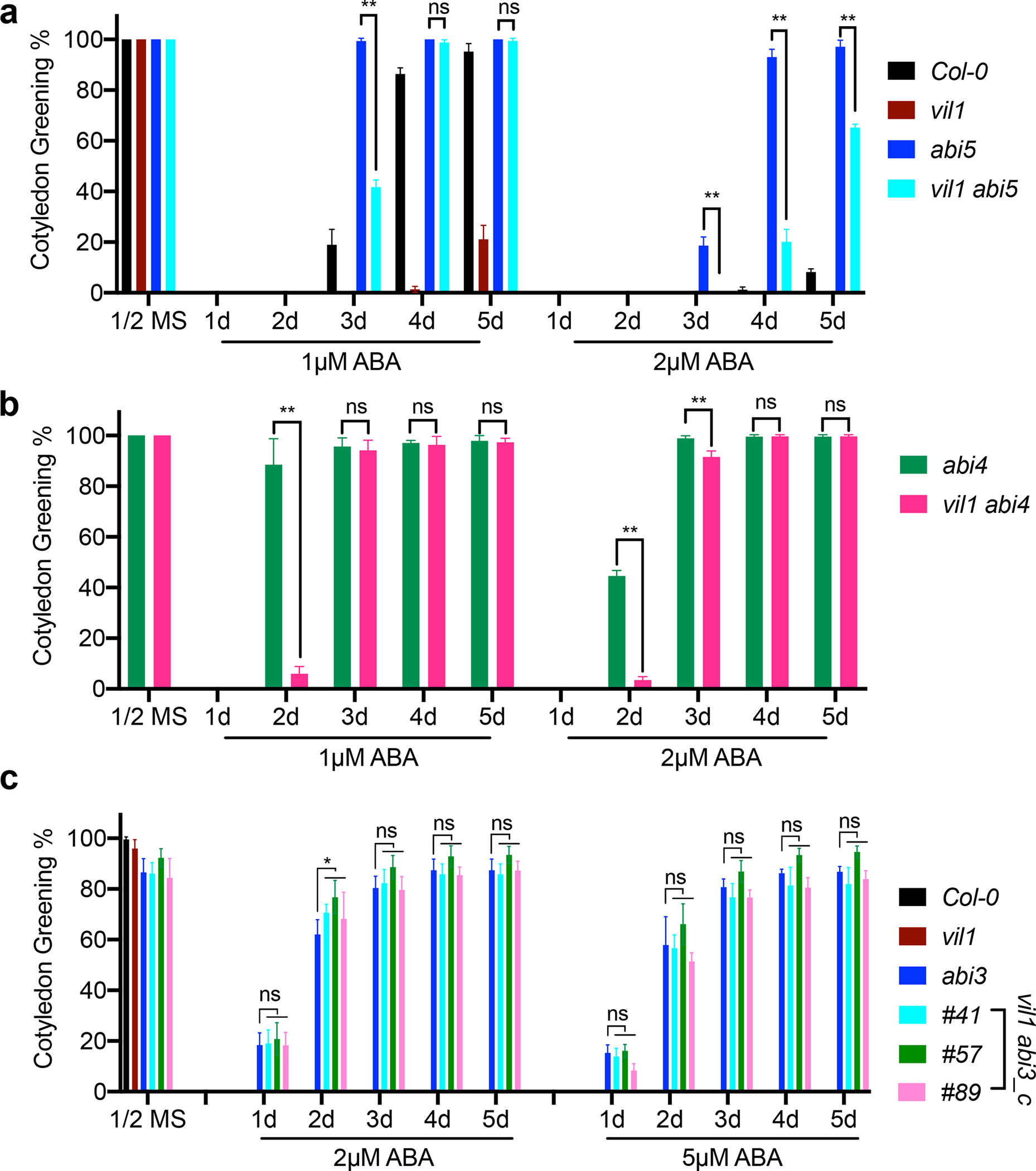
VIL1 regulates the ABA response through ABI3 and ABI4 in Arabidopsis. (a and b) ABA response of indicated genotypes. Green cotyledon percentage of seeds grown on 1/2 MS medium for 3 days or 1/2 MS medium containing 1 μM ABA or 2 μM ABA for indicated days were presented. Values are mean ± s. d. (n = 3). One-way ANOVA Tukey’s multiple comparison test was conducted; asterisks indicate P < 0.01 of distinct groups, ns indicates no significant differences of distinct groups. (c) ABA response of WT, vil1, abi3 and vil1 abi3_c double mutants. Green cotyledon percentage of seeds grown on 1/2 MS medium for 3 days or 1/2 MS medium containing 2 μM ABA or 5 μM ABA for indicated days were presented. Error bars: ± s. d. (n = 3). One-way ANOVA Tukey’s multiple comparison test was conducted; asterisks indicate P < 0.05 of distinct groups, ns indicate no significant differences of distinct groups.
As VIL1 and ABI3 are located very close to each other in the Arabidopsis genome, we could not isolate the vil1 abi3 double mutant by genetic crossings. Therefore, we mutated ABI3 by CRISPR/Cas9 method (Liu et al., 2017) in vil1 mutant background to create vil1 abi3_c double mutants (Fig. S8a,b). Consistent with previously reported abi3 deletion mutant alleles (Nambara et al., 1994), all confirmed CRISPR/Cas9-generated mutants produce green seeds that are intolerant to desiccation (Fig. S8c). To validate the genetic relationship between VIL1 and ABI3, three independent CRISPR/Cas9 lines of vil1 abi3_c double mutants (#41, #51, and #89) were selected and compared their responses to the ABA treatment with abi3 and vil1 single mutants (Fig. 6c, S8c). Interestingly, the germination rate of vil1 abi3_c double mutants show no difference compared to abi3 single mutant even at higher ABA concentrations (Fig. 6c), suggesting that abi3 is completely epistatic to vil1. Therefore, our genetic analysis, combined with the de-repression of ABI3 and ABI4 observed in vil1 mutants, supports that VIL1 promotes seed germination through the deposition of H3K27me3 at ABI3 and ABI4 chromatin to repress their expressions.
vil1 mutants exhibit enhanced drought tolerance
Given that the vil1 mutant is hypersensitive to ABA treatment at the early germination stage, we also addressed whether VIL1 may be involved in other stress responses. ABA is a key phytohormone in controlling water usage in plants (Cutler et al., 2010; Chen, K et al., 2020; Zhang et al., 2020). Therefore, we examined the effect of vil1 mutations on drought response. Surprisingly, vil1 mutants had an increased survival rate under drought stress than WT (Fig. 7a). The drought tolerance phenotype observed in vil1 mutants is consistent with the ABA hypersensitivity observed during seed germination in vil1 mutants. Consistent with the drought tolerance observed in vil1 mutants, water loss assays using detached leaves also showed that vil1 mutants have much lower water-loss rates than WT and the complementation line (gVIL1) (Fig. 7b, S9a). Similar results were observed in dehydration stress assays, where mutations in VIL1 resulted in an improved tolerance to dehydration stress (Fig. S9b,c).
Dehydration condition triggers the induction of several stress-related genes, including the dehydration-responsive gene, RD29A (Nakashima et al., 2006). We also identified RD29A among DEGs in vil1 mutants (Table S2). Indeed, RD29A is induced at a much higher level in vil1 mutant background under dehydration condition compared to WT upon dehydration condition (Fig. 7c), indicating that VIL1 functions to limit the induction of RD29A under drought conditions. This is similar to the de-repression of ABI3, and ABI4 observed in response to the ABA treatment in vil1 mutants. Therefore, we determined whether RD29A chromatin is also a direct target of VIL1 (Fig. 7e,f). Indeed, VIL1 directly associates with RD29A chromatin. However, the dehydration condition does not change the level of VIL1 enrichment at RD29A (Fig. 7g). We also determined the enrichment of H3K27me3 at RD29A, but the level of H3K27me3 does not change by the dehydrating treatment (Fig. 7h). However, the level of H3K27me3 at RD29A is reduced in vil1 mutants (Fig. 7h), indicating that the reduced level of H3K27me3 contributes to the increased induction of RD29A upon dehydration in vil1 mutants.
Discussion
Previous studies showed that PHD-finger protein VIL1 is a facultative subunit of VRN2-PRC2 and is required for the repression of FLC in response to winter cold (Sung et al., 2006; Greb et al., 2007; De Lucia et al., 2008). Similar facultative subunits of PRC2 are present in other eukaryotes, such as Pcl-PRC2 of Drosophila and PHF1-PRC2 of animals (Hennig & Derkacheva, 2009). In vitro studies have shown that the mammalian PHD finger protein, PHF1, promotes the ability of EZH2 to catalyze H3K27me3 of its target chromatin (Sarma et al., 2008). Similarly, VIL1-PRC2 directly associates with FLC and is necessary for the H3K27me3 enrichment at the FLC chromatin by vernalization (Sung et al., 2006; Greb et al., 2007). Two E(z) homologs, CLF and SWN, are functionally redundant in PRC2 complexes in the sporophyte (Chanvivattana et al., 2004) and are necessary for proper development. The clf swn double mutant is pleiotropic, and its development is arrested at early seedling stages, indicating the involvement of PRC2 in a wide range of developmental processes (Chanvivattana et al., 2004; Hennig & Derkacheva, 2009; Farrona et al., 2011; Mozgova & Hennig, 2015; Shu et al., 2019). On the other hand, mutations in facultative components of PRC2, such as VIL1, compromise only subsets of PRC2-regulated loci, as shown in this study.
It has been reported that the conditional fie mutants exhibit enhanced dormancy and germination defects, indicating the possible roles of PRC2 in the ABA response (Bouyer et al., 2011). The conditional fie mutants also caused severe growth defects, similar to clf swn double mutants (Bouyer et al., 2011). Therefore, it is difficult to address whether the defects shown in the early seedling stage reflect the direct involvement of FIE or the secondary effect due to the pleiotropic phenotypes observed in fie mutants. A study using the weak double mutant clf-50 swn-1, in which swn-1 is a hypomorphic allele, accelerated leaf senescence by the ABA treatment in Arabidopsis, a hyposensitive response to the ABA treatment (Liu et al., 2019). The CLF/SWN-mediated H3K27me3 limits the ABA-induced senescence-associated genes and thus delays the leaf senescence (Liu et al., 2019). It has also been reported that the clf mutant is more sensitive to dehydration treatment, implying the hyposensitive response to the ABA treatment in clf mutants (Liu et al., 2014). Therefore, the role of core Polycomb in the ABA responses is somewhat complicated in part due to developmental defects observed in Polycomb mutants.
However, vil1 mutants do not exhibit severe developmental defects other than hypersensitivity to the ABA treatment during early seedling development. The level of endogenous ABA rapidly declines after germination (Weitbrecht et al., 2011), and the ABA-responsive genes, such as ABI3, ABI4, and ABI5, are quickly repressed after germination. We show that VIL1 directly associates with two ABA-signaling upstream transcription factor loci, ABI3 and ABI4, to repress them effectively by facilitating H3K27me3 during early seedling formation (Fig. 2). In addition, the ABA-induced expression of ABI3 and ABI4 also include the rapid reduction of the enrichment of VIL1 and H3K27me3 (Fig. 2 and Fig. 3), indicating the dynamic roles of VIL1-mediated H3K27me3 in the ABA-mediated transcriptional regulation. However, there is no rapid reduction in the enrichment of core PRC2 components in response to ABA, suggesting that Polycomb utilizes a facultative component to fine-tune its chromatin remodeling activity. Therefore, our work demonstrates that a facultative component of PRC2, such as VIL1, provides a flexible regulatory module to control its chromatin-modifying activity in response to developmental and hormonal signals.
Upon the deprivation of ABA, inactive SnRK2 would likely diminish the pool of activating transcription factors to initiate transcriptional cascades. In parallel, our study shows that VIL1-PRC2 functions to ensure the proper repression of ABA-responsive genes during early seedling establishment by directly mediating the deposition of a repressive histone modification, H3K27me3, at ABI3 and ABI4 (Fig. S10). Although the reduction in H3K27me3 levels at ABI3 and ABI4 correlates with the eviction of VIL1 (Fig. 2c,d), the degree of H3K27me3 reduction by ABA treatment is more severe than in vil1 mutants (Fig. 3a,b). This suggests that there are other factors contributing to the reduction of H3K27me3 by ABA. It remains to be determined whether ABA-signaling components directly dictate recruitment and/or eviction of VIL1 and/or other Polycomb proteins.
Many stress-induced genes have been targeted for the potential candidate genes to improve tolerance to stress conditions in plants by genetic engineering. Over- and/or under-expression of such genes have shown to effectively improve resistance to certain stress conditions (Zhang et al., 2020). However, simple constitutive expression of stress-induced genes often results in developmental or growth defects (Martignago et al., 2019). Therefore, inducible promoter-driven strategies have been most successful (Kasuga et al., 1999). It should be noted that vil1 mutants do not show extreme ectopic expression of stress-responsive genes in the absence of stimuli (Table S2). Instead, VIL1-mediated H3K27me3 appears to limit the degree of induction of RD29A under drought conditions (Fig. 7e). Therefore, a mild but significant increase in the level of transcriptional induction of RD29A results in unexpected drought tolerance without compromising the growth of plants in vil1 mutants.
Supplementary Material
Acknowledgment
We thank Dr. Yuhai Cui (Western University, Canada) for pCLF:CLF-GFP/clf29 (gCLF-GFP) and pSWN:SWN-GFP/swn-4 (gSWN-GFP) seeds. This work was supported by NIH R01GM100108, NSF IOS 1656764 to S. S., and NIH R01GM115879 to H. Q.
Footnotes
Supplementary Information
Table S1. Primers used in this study.
Table S2. Differentially expressed gene in vil1.
Fig. S1 Differentially expressed genes in vil1 mutants.
Fig. S2 VIL1 regulates ABA response in Arabidopsis.
Fig. S3 VIL1 represses ABI3 and ABI4 expression during germination.
Fig. S4 The H3K27me3 levels at ABI5 locus during seed germination.
Fig. S5 VIL1 expressions are not regulated by ABA treatment.
Fig. S6 CLF and SWN directly bind to ABI3 and ABI4 but not to ABI5.
Fig. S7 VIL1 functions together with PRC2 components to regulate the ABA response.
Fig. S8 Seed phenotype of Col-0, vil1, abi3, and vil1 abi3 double mutants in Arabidopsis.
Fig. S9 Water loss assay in Arabidopsis.
Fig. S10 Working model showing that VIL1-PRC2 regulates seed germination in Arabidopsis.
Data Availability
The data discussed in this publication have been deposited in NCBI Gene Expression Omnibus and are accessible through GEO series accession no. GSE180587.
Reference
- Baroux C, Gagliardini V, Page DR, Grossniklaus U. 2006. Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20(9): 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi F, Cordoba E, Dupre P, Mendoza MS, Roman CS, Leon P. 2009. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59(3): 359–374. [DOI] [PubMed] [Google Scholar]
- Bouyer D, Roudier F, Heese M, Andersen ED, Gey D, Nowack MK, Goodrich J, Renou JP, Grini PE, Colot V, et al. 2011. Polycomb repressive complex 2 controls the embryo-to-seedling phase transition. PLoS Genet 7(3): e1002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakov VP, Wu HC, Jinn TL. 2019. Coordination of ABA and Chaperone Signaling in Plant Stress Responses. Trends Plant Sci 24(7): 636–651. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran U, Luo X, Zhou W, Shu K. 2020. Multifaceted Signaling Networks Mediated by Abscisic Acid Insensitive 4. Plant Commun 1(3): 100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon YH, Sung ZR, Goodrich J. 2004. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131(21): 5263–5276. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. 2020. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol Plant 13(8): 1194–1202. [DOI] [PubMed] [Google Scholar]
- Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y. 2020. Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62(1): 25–54. [DOI] [PubMed] [Google Scholar]
- Ciferri C, Lander GC, Maiolica A, Herzog F, Aebersold R, Nogales E. 2012. Molecular architecture of human polycomb repressive complex 2. Elife 1: e00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6): 735–743. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679. [DOI] [PubMed] [Google Scholar]
- De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. 2008. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A 105(44): 16831–16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkacheva M, Steinbach Y, Wildhaber T, Mozgova I, Mahrez W, Nanni P, Bischof S, Gruissem W, Hennig L. 2013. Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J 32(14): 2073–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrona S, Thorpe FL, Engelhorn J, Adrian J, Dong X, Sarid-Krebs L, Goodrich J, Turck F. 2011. Tissue-specific expression of FLOWERING LOCUS T in Arabidopsis is maintained independently of polycomb group protein repression. Plant Cell 23(9): 3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CZ, Chen Y, Wang C, Kong YH, Wu WH, Chen YF. 2014. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J 80(4): 654–668. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. Plant Cell 14 Suppl: S15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. 2000. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12(4): 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. 1998. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10(6): 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. 2009. In vitro reconstitution of an abscisic acid signalling pathway. Nature 462(7273): 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall AR, Levy YY, Wilson A, Dean C. 2001. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107(4): 525–535. [DOI] [PubMed] [Google Scholar]
- Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. 1992. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4(10): 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C. 2007. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol 17(1): 73–78. [DOI] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV. 2005. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8(2): 183–187. [DOI] [PubMed] [Google Scholar]
- Hennig L, Derkacheva M. 2009. Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet 25(9): 414–423. [DOI] [PubMed] [Google Scholar]
- Jia H, Suzuki M, McCarty DR. 2014. Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks. Wiley Interdiscip Rev Dev Biol 3(1): 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. 1999. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17(3): 287–291. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sung S. 2013. Coordination of the Vernalization Response through a VIN3 and FLC Gene Family Regulatory Network in Arabidopsis. Plant Cell 25(2): 454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Bordiya Y, Kathare PK, Zhao B, Zong W, Huq E, Sung S. 2021. Phytochrome B triggers light-dependent chromatin remodelling through the PRC2-associated PHD finger protein VIL1. Nat Plants 7(9):1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee J, Eshed-Williams L, Zilberman D, Sung ZR. 2012. EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet 8(3): e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Seo PJ. 2019. MYB96 recruits the HDA15 protein to suppress negative regulators of ABA signaling in Arabidopsis. Nat Commun 10(1): 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Cheng J, Zhuang Y, Ye L, Li Z, Wang Y, Qi M, Xu L, Zhang Y. 2019. Polycomb repressive complex 2 attenuates ABA-induced senescence in Arabidopsis. Plant J 97(2): 368–377. [DOI] [PubMed] [Google Scholar]
- Liu H, Ding Y, Zhou Y, Jin W, Xie K, Chen LL. 2017. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol Plant 10(3): 530–532. [DOI] [PubMed] [Google Scholar]
- Liu N, Fromm M, Avramova Z. 2014. H3K27me3 and H3K4me3 chromatin environment at super-induced dehydration stress memory genes of Arabidopsis thaliana. Mol Plant 7(3): 502–513. [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. 2001. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci U S A 98(8): 4782–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. 2002. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32(3): 317–328. [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. 2009. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324(5930): 1064–1068. [DOI] [PubMed] [Google Scholar]
- Martignago D, Rico-Medina A, Blasco-Escamez D, Fontanet-Manzaneque JB, Cano-Delgado AI. 2019. Drought Resistance by Engineering Plant Tissue-Specific Responses. Front Plant Sci 10: 1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgova I, Hennig L. 2015. The Polycomb Group Protein Regulatory Network. Annual Review of Plant Biology, Vol 66 66: 269–296. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Katsura K, Maruyama K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2006. Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol Biol 60(1): 51–68. [DOI] [PubMed] [Google Scholar]
- Nambara E, Keith K, McCourt P, Naito S. 1994. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol 35(3): 509–513. [PubMed] [Google Scholar]
- Parcy F, Valon C, Kohara A, Misera S, Giraudat J. 1997. The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9(8): 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324(5930): 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirats-Llobet M, Han SK, Gonzalez-Guzman M, Jeong CW, Rodriguez L, Belda-Palazon B, Wagner D, Rodriguez PL. 2016. A Direct Link between Abscisic Acid Sensing and the Chromatin-Remodeling ATPase BRAHMA via Core ABA Signaling Pathway Components. Mol Plant 9(1): 136–147. [DOI] [PubMed] [Google Scholar]
- Perruc E, Kinoshita N, Lopez-Molina L. 2007. The role of chromatin-remodeling factor PKL in balancing osmotic stress responses during Arabidopsis seed germination. Plant J 52(5): 927–936. [DOI] [PubMed] [Google Scholar]
- Sarma K, Margueron R, Ivanov A, Pirrotta V, Reinberg D. 2008. Ezh2 requires PHF1 to efficiently catalyze H3 lysine 27 trimethylation in vivo. Mol Cell Biol 28(8): 2718–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, Chen C, Thapa RK, Bian S, Nguyen V, Yu K, Yuan ZC, Liu J, Kohalmi SE, Li C, et al. 2019. Genome-wide occupancy of histone H3K27 methyltransferases CURLY LEAF and SWINGER in Arabidopsis seedlings. Plant Direct 3(1): e00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Schmitz RJ, Amasino RM. 2006. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev 20(23): 3244–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. 2017. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res 45(W1): W122–W129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, Parcy F. 2006. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18(7): 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu C, Cheng J, Liu J, Zhang L, He C, Shen WH, Jin H, Xu L, Zhang Y. 2016. Arabidopsis Flower and Embryo Developmental Genes are Repressed in Seedlings by Different Combinations of Polycomb Group Proteins in Association with Distinct Sets of Cis-regulatory Elements. PLoS Genet 12(1): e1005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ. 2015. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitbrecht K, Muller K, Leubner-Metzger G. 2011. First off the mark: early seed germination. J Exp Bot 62(10): 3289–3309. [DOI] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JR, Lee CH, Oksuz O, Stafford JM, Reinberg D. 2019. PRC2 is high maintenance. Genes & Development 33(15–16): 903–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao Y, Zhu JK. 2020. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev Cell 55(5): 529–543. [DOI] [PubMed] [Google Scholar]
- Zong W, Zhao B, Xi Y, Bordiya Y, Mun H, Cerda NA, Kim DH, Sung S. 2021. DEK domain-containing proteins control flowering time in Arabidopsis. New Phytol. 231 (1), 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data discussed in this publication have been deposited in NCBI Gene Expression Omnibus and are accessible through GEO series accession no. GSE180587.


