Abstract
Background
Acute kidney injury (AKI) after transcatheter aortic valve replacement (TAVR) is associated with increased mortality. However, it is controversial whether AKI affects prognosis per se, being linked to baseline chronic kidney disease (CKD) and bleeding complications. The aim of this study was to disentangle, applying mediation analysis, the association between AKI and clinical outcome, considering CKD and bleedings.
Methods and Results
Consecutive patients undergoing TAVR were prospectively enrolled at 5 high‐volume centers in Italy. AKI was defined according to Valve Academic Research Consortium‐3 consensus, whereas bleeding with Bleeding Academic Research Consortium. Primary outcome was all‐cause mortality after 1‐year follow‐up. Among 2621 patients undergoing TAVR, AKI occurrence was associated with 1‐year mortality. This association of AKI with the primary end points remained significant after adjusting for baseline risk estimators, either Society of Thoracic Surgeons score (hazard ratio [HR], 2.78 [95% CI, 1.95–3.80], P<0.001) or EuroSCORE‐II (HR, 1.85 [95% CI, 1.35–2.56], P<0.001). Both AKI and CKD significantly and independently affected primary outcome (HR, 3.06 [95% CI, 2.01–4.64], P<0.001 and HR, 1.82 [95% CI 1.27–2.65], P<0.01, respectively). The estimated proportion of the total effect of CKD mediated via AKI was, on average, 15%, 95% CI, 4%–29%, P<0.001. The significant effect of Bleeding Academic Research Consortium 2–5 bleedings on the primary outcome was not mediated by AKI.
Conclusions
AKI occurs in 1 out of 6 patients and significantly mediates one fifth of the effect of baseline CKD on all‐cause mortality after TAVR. Our analysis supports a systematic effort to prevent AKI during TAVR, which may potentially translate into improved patients' 1‐year survival.
Keywords: acute kidney injury, complications, transcatheter aortic valve replacement
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- AKI
acute kidney injury
- BARC
Bleeding Academic Research Consortium
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
Transcatheter aortic valve replacement is increasingly becoming a mainstay in the treatment of patients with aortic stenosis.
Acute kidney injury (AKI) is a relative common complication of patients undergoing transcatheter aortic valve replacement and associated with poor outcome; however, the incidence of AKI is more frequent in patients with baseline chronic kidney disease and may also be mutually associated with transcatheter aortic valve replacement related bleeding complications; therefore, it is not clear which and how much is the individual contribution on outcome of AKI over the baseline patient risk.
With multivariable mediation analysis we aimed to disentangle this complex clinical puzzle, we showed that AKI is individually responsible for one fifth of the clinical impact of stage 4 to 5 chronic kidney disease considering also bleeding complications.
What Are the Clinical Implications?
This implies that preventive measures aimed to reduce AKI incidence might eventually improve outcome with a sizable number of patients needed to save 1 life.
Transcatheter aortic valve replacement (TAVR) has become the standard of care for patients with severe symptomatic aortic stenosis who are at intermediate and high risk for surgery. 1 , 2 As TAVR is also becoming an attractive therapeutic option for patients at lower surgical risk, prompt recognition and management of intra‐ and periprocedural complications become pivotal. Acute kidney injury (AKI) is frequently found in patients following TAVR and it is associated with increased morbidity and mortality. 3 , 4 , 5
The most important risk factor for AKI, in patients receiving iodinated contrast, 6 is reduced renal function because of chronic kidney disease (CKD), 7 , 8 a strong predictor of long‐term mortality after TAVR. 9 , 10 , 11 Therefore, it is controversial whether AKI, a potentially preventable complication of TAVR, causally mediates the virtually unmodifiable impact of CKD on clinical outcome. Likewise, bleeding complications increase the risk of AKI and impact on mortality after TAVR. 3 , 12 , 13 A recent analysis of competing risks in patients undergoing percutaneous coronary intervention for acute coronary syndrome, showed that the mortality benefit of radial approach linked to AKI limiting was superior to the effect mediated by bleeding reduction, 14 suggesting that AKI might be the Cardan joint between baseline risk profile and clinical outcome in different clinical scenarios.
The aim of this study was to disentangle, applying mediation analysis, the association between AKI and clinical outcome, considering baseline renal function and bleedings.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Consecutive patients undergoing successful TAVR were prospectively enrolled in local clinical registries of 5 high‐volume centers in Italy: IRCCS Ospedale Policlinico San Martino (Genoa), IRCCS Policlinico San Donato (Milan), Città Della Salute e della Scienza, (Turin), Ospedale Niguarda Ca′ Granda (Milan), and Magna Graecia University (Catanzaro), between January 2014 to December 2019.
Individual patient‐level data were merged in an ad hoc database; we excluded patients who died within 24 hours from TAVR and patients who did not have a serum creatinine (SCr) assessment at ≥48 hours; we also excluded patients on permanent hemodialysis at baseline.
All patients signed an informed consent allowing the use of their anonymized clinical information for medical research purposes, as approved by the local (Genova, Italy) institutional review board. The study complies with the Declaration of Helsinki.
Study Protocol and Definitions
Patients underwent TAVR according to standard of care. 15 , 16 Both balloon expandable and the self‐expandable valves were used. The femoral artery was mainly approached percutaneously using a preclosure technique. Prosthesis size was determined with computed tomographic scan. Rapid right ventricular pacing (range 160–200 beats/min) was generally performed during balloon dilation for native aortic valves or implanted bioprosthetic valves. Iodixanol (Visipaque, GE Healthcare, Buc, France), iohexol (Omnipaque, GE Healthcare), or iomeprol (Iomeron, Bracco, Milano, Italy) were used during TAVR procedure. Cardiac catheterization or any other significant examination requiring dye administration use were avoided for 72 hours before TAVR.
SCr was measured at baseline and until discharge daily. Clinical assessment was scheduled at 1 and 6 months after the procedure and at 1 year of follow‐up. Glomerular filtration rate (GFR) was estimated (e) with Modification of Diet in Renal Disease study equation. 17 AKI was defined according to the Kidney Disease Improving Global Outcome classification 18 as recommended by Valve Academic Research Consortium 3 consensus. 19
Briefly, stage 1 AKI was defined as increase in SCr >0.3 mg/dL or >1.5 to 2x above baseline. An increase in SCr >2 to 3x above baseline defined stage 2 AKI, whereas an increase in SCr >3x above baseline or baseline SCr of 4 mg/dL with an increase of 0.5 mg/dL, defined stage 3 AKI.
Primary outcome measure was all‐cause death after 1 year of follow‐up. In‐hospital stroke, vascular complications, myocardial infarction were defined according to the Valve Academic Research Consortium 3 consensus 19 ; bleeding events were defined according to the Bleeding Academic Research Consortium (BARC). 20
Statistical Analysis
Categorical variables were expressed as count (percentage) and compared with the χ2 test; continuous variables were expressed as mean (SD) or median (interquartile range) and compared with the Student t test, analysis of variance (ANOVA) or the respective nonparametric test according to distribution.
The hazard ratios (HRs) and 95% CI of AKI, bleeding complications, and baseline CKD on 1‐year all‐cause mortality were estimated by fitting Cox proportional hazard regression models. We used the Society of Thoracic Surgeons (STS) and logistic EuroSCORE II, both universally accepted baseline risk stratifiers for HR esteem adjustment.
Next, we performed mediation analysis to elucidate the association between AKI; CKD; BARC 2, 3, or 5 bleeding complications; and the primary outcome. 14 , 21 , 22
Briefly, mediation analysis consists of a multivariable analysis that permits us to disentangle and quantify the precise role of the direct and indirect effects of a mediator on the association between a predictor and an outcome.
Firstly, we explored the relationship between baseline estimated GFR (eGFR) as independent variable and 1‐year mortality. We fitted a restricted cubic splines model with 4 knots; the same approach was used between baseline eGFR and 1‐year mortality; contrast dose was included in the latter model for risk adjustment.
The formal mediation analysis had 3 steps. 21 , 23 First, we used logistic regression to determine whether the baseline eGFR (cutoff was set at 30 mL/min per 1.73 m2, corresponding to CKD stage 4–5), was significantly associated with the primary outcome measure and AKI (as a mediator). We then fitted a logistic regression model, which included both baseline eGFR and AKI as predictors of primary outcome. Finally, we used a bootstrap of 5000 randomly derived samples to estimate the odds ratio (OR) and 95% CI of the AKI effect on the association between baseline eGFR and primary outcome. 24 We report the relationship between the baseline eGFR and AKI and 1‐year mortality using OR, the natural logs of which were equal to the beta coefficients. To gauge the magnitude of the effect of AKI on the association with baseline eGFR with the primary outcome, we also estimated the proportion of mediated effect, which was calculated as the indirect effect (eg, effect of baseline eGFR on primary outcome related to AKI) divided by the sum of the direct (eg, direct effect of baseline eGFR on primary outcome) and indirect effect. The occurrence of BARC 2, 3, or 5 bleeding complication and contrast dose (by quartiles) were included as covariates for risk adjustment into the models for mediation analysis. We performed several sensitivity analyses of this approach to verify the consistency of our results: first, we included only patients who received fully percutaneous TAVR, therefore excluding surgical accesses; second, we included only stage 2 and 3 (more severe) AKI events; third, we used a softer baseline eGFR cutoff of 45 mL/min per 1.73 m2; finally, we explored the variable of BARC 2, 3, or 5 bleeding events as mediator, while keeping into the model the same covariates.
A P value lower than 0.05 was considered statistically significant; data were managed and analyzed in R environment 3.6.2 “dark and stormy night” (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
A total of 2696 consecutive patients undergoing TAVR were included (Figure 1). We excluded 34 patients who died within 24 hours of TAVR (of whom 7 experienced bleeding complications), 15 patients on chronic hemodialysis, and 26 patients with missing SCr values, leading to a final population of 2621 patients. Our sample included 1209 (46.1%) men, and mean age was 82±6.4 years. Median eGFR was 61.1 [45.7, 77.1] mL/min per 1.73 m2 in patients without AKI and 50.6 [35.0, 68.9] mL/min per 1.73 m2 in patients with AKI, P<0.001. STS score and EuroSCORE II values were 6.6 [4.0, 11.9] and 4.9 [3.1, 8.1], respectively, with higher values in AKI compared with no AKI. All baseline characteristics are depicted in Table 1, whereas procedural variables are shown in Table 2.
Figure 1. Patient's flow.

AKI indicates acute kidney injury; BARC, Bleeding Academic Research Consortium; and TAVR, transcatheter aortic valve replacement.
Table 1.
Baseline Population Characteristics
| No AKI | AKI | P value | |
|---|---|---|---|
| No. (%) | 2169 (82.7) | 452 (17.3) | |
| Age, y | 82.1 (6.5) | 81.8 (6.3) | 0.319 |
| Male sex | 986 (45.5) | 223 (49.3) | 0.146 |
| Body mass index, kg/m2 | 25.4 (22.9–28.4) | 25.4 (23.1–28.1) | 0.816 |
| History of coronary artery disease | 750 (34.9) | 169 (37.6) | 0.291 |
| New York Heart Association class | 0.008 | ||
| 1 | 44 (2.0) | 9 (2.0) | |
| 2 | 456 (21.1) | 121 (26.8) | |
| 3 | 1550 (71.7) | 289 (63.9) | |
| 4 | 111 (5.1) | 33 (7.3) | |
| Chronic obstructive pulmonary disease | 419 (19.4) | 90 (19.9) | 0.856 |
| Diabetes | 608 (28.1) | 146 (32.3) | 0.083 |
| Prior myocardial infarction | 419 (19.4) | 85 (18.8) | 0.838 |
| Any prior valve procedures | 140 (6.5) | 26 (5.8) | 0.639 |
| Baseline hemoglobin, g/dL | 12.0 (10.8–13.2) | 11.5 (10.4–12.6) | <0.001 |
| N‐terminal pro‐B‐type natriuretic peptide, ng/mL | 2917.0 (1279.0–6218.0) | 4433.5 (1568.2–9560.5) | 0.002 |
| Serum creatinine (mg/dL) baseline | 1.1 (0.8–1.3) | 1.2 (0.9–1.8) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 61.1 (45.7–77.1) | 50.6 (35.0–68.9) | <0.001 |
| eGFR <30, mL/min per 1.73 m2 | 137 (6.3) | 93 (20.6) | <0.001 |
| eGFR <45, mL/min per 1.73 m2 | 511 (23.6) | 180 (39.8) | <0.001 |
| Atrial fibrillation | 713 (33.3) | 157 (35.2) | 0.481 |
| Left ventricular ejection fraction (%) | 53.9 (11.4) | 52.6 (11.7) | 0.039 |
| Logistic EuroSCORE II | 6.3 (3.9–11.4) | 7.2 (4.4–13.0) | 0.017 |
| Society of Thoracic Surgeons score | 4.8 (3.0–7.8) | 5.5 (3.5–9.4) | 0.005 |
AKI indicates acute kidney injury; and eGFR, estimated glomerular filtration rate.
Table 2.
TAVR Procedural Characteristics
| No AKI | AKI | P value | |
|---|---|---|---|
| No. (%) | 2169 (82.7) | 452 (17.3) | |
| LV‐aortic max gradient, mm Hg | 78.6 (24.7) | 79.9 (25.4) | 0.334 |
| LV‐aortic mean gradient, mm Hg | 47.8 (14.9) | 47.5 (15.6) | 0.699 |
| TAVR valve in valve | 77 (3.6) | 17 (3.8) | 0.936 |
| Balloon expandable valve | 582 (26.8) | 151 (33.4) | 0.006 |
| Procedural access | <0.001 | ||
| Transfemoral | 1662 (89.0) | 311 (83.2) | |
| Transsubclavian | 131 (7.0) | 30 (8.0) | |
| Transapical | 59 (3.2) | 28 (7.5) | |
| Transcarotid | 16 (0.9) | 4 (1.1) | |
| Transcaval | 0 (0.0) | 1 (0.3) | |
| Any surgical access | 306 (17.0) | 88 (25.1) | <0.001 |
| Valve type | <0.001 | ||
| Sapien XT | 91 (4.2) | 15 (3.3) | |
| Sapien 3 | 447 (20.6) | 117 (25.9) | |
| Sapien 3 ultra | 52 (2.4) | 21 (4.6) | |
| Corevalve–evolute | 878 (40.5) | 203 (44.9) | |
| Lotus | 311 (14.3) | 62 (13.7) | |
| Symetis–accurate neo | 144 (6.6) | 13 (2.9) | |
| Allegra | 6 (0.3) | 2 (0.4) | |
| Portico | 195 (9.0) | 18 (4.0) | |
| Direct flow | 45 (2.1) | 1 (0.2) | |
| Predilatation | 708 (32.7) | 165 (36.5) | 0.128 |
| Postdilatation | 682 (31.4) | 133 (29.4) | 0.431 |
| Procedural time | 84.0 (63.0–120.0) | 103.0 (75.2–143.8) | <0.001 |
| Contrast dose, mL | 196.5 (95.0) | 227.4 (102.0) | <0.001 |
| Ranked contrast dose, mL | <0.001 | ||
| <100 | 149 (9.0) | 13 (3.9) | |
| 100–149 | 389 (23.6) | 49 (14.6) | |
| 150–199 | 417 (25.3) | 88 (26.2) | |
| >200 | 693 (42.1) | 186 (55.4) | |
| Paravalvular leak | 0.001 | ||
| Absent‐trivial | 803 (47.6) | 204 (58.8) | |
| 1+ | 762 (45.1) | 117 (33.7) | |
| 2+ | 113 (6.7) | 24 (6.9) | |
| 3+ | 10 (0.6) | 2 (0.6) | |
| Hospitalization length, d | 8.0 (5.0–11.2) | 11.0 (8.0–19.0) | <0.001 |
| Serum creatinine (mg/dL) post TAVR | 1.0 (0.8–1.3) | 1.8 (1.4–2.9) | <0.001 |
AKI indicates acute kidney injury; LV, left ventricular; and TAVR, transcatheter aortic valve replacement.
AKI and All‐Cause Mortality
AKI occurred in 452 patients (17.3%), of whom 381 (84.2%) had stage 1, 41 (9.1%) stage 2, and 63 (13.9%) stage 3 AKI (Table 2).
Patients with AKI had significantly higher all‐cause mortality at 30 days and after 1‐year follow‐up (Figure 2 and Table 3), with a crude HR of 2.18 (95% CI, 1.65–2.86, P<0.001), which was still significant after adjusting for STS 2.78 (95% CI, 1.95–3.80, P<0.001) or EuroSCORE II 1.85 (95% CI, 1.35–2.56, P<0.001). We also found a statistically significant gradient of effect between stage 2 to 3 AKI and primary outcome as compared with stage 1 AKI and no AKI (Figure S1 through S4).
Figure 2. Cumulative all‐cause mortality in patients stratified for AKI occurring after TAVR.

AKI indicates acute kidney injury defined in accordance with the Valve Academic Research Consortium‐3 consensus document; and TAVR, transcatheter aortic valve replacement.
Table 3.
TAVR‐Related Clinical Events
| No AKI | AKI | P value | |
|---|---|---|---|
| No. (%) | 2169 (82.7) | 452 (17.3) | |
| All‐cause mortality at 30‐d | 54 (2.5) | 23 (5.1) | 0.005 |
| All‐cause mortality at 1‐y follow‐up | 174 (8.0) | 72 (15.9) | <0.001 |
| Cardiovascular‐cause mortality | 91 (4.1) | 37 (8.1) | <0.001 |
| AKI | 452 (100.0) | <0.001 | |
| Stage AKI | <0.001 | ||
| 1 | 348 (77.0) | ||
| 2 | 41 (9.1) | ||
| 3 | 63 (13.9) | ||
| Bleeding complications (Bleeding Academic Research Consortium 20 2, 3, or 5) | 456 (21.6) | 145 (32.9) | <0.001 |
Mortality was assessed at 1‐year follow‐up, other events were assessed in‐hospital. AKI indicates acute kidney injury; and TAVR, transcatheter aortic valve replacement.
Bleedings, CKD, and All‐Cause Mortality
There were 609 (23%) bleeding complications (BARC 2, 3, or 5), after excluding 7 events occurring in patients within 24 hours of TAVR and were significantly associated with poor 30‐day and 1‐year all‐cause mortality (Table 3 and Figure S2); STS and EuroSCORE II‐adjusted HR for the association of BARC 2, 3, and 5 bleedings on primary outcome were respectively 1.76 (95% CI, 1.27–2.44, P<0.001) and 1.65 (95% CI, 1.18–2.29, P<0.001).
CKD stage 3b‐5 (eGFR <45 mL/min per 1.73 m2) was found in 691 (26.4%) of patients, whereas CKD stage 4 to 5 (eGFR <30 mL/min per 1.73 m2) was found in 230 (8.8%) of patients. After 1‐year follow‐up, 44 (19.1%) patients with CKD died compared with 202 (8.4%) patients without CKD (Figure S3). Higher CKD stages were associated with statistically significant unadjusted (Figures S3 and S4) and STS/EuroSCORE II‐adjusted increased risk of 1‐year mortality, with HRs, respectively, of 1.71 (95% CI, 1.25–2.35, P<0.001) and 1.54 (95% CI, 1.12–2.13, P<0.001) for stage 3 and 1.95 (95% CI, 1.32–2.87, P<0.001) and 2.06 (95% CI, 1.37–3.12, P<0.001) for stage 4 to 5 CKD.
Effect of Baseline eGFR on AKI and 1‐Year Outcome
We explored the interplay between baseline eGFR and AKI with restricted cubic splines, showing that there was an j‐curve relationship with a flat, low risk (OR ≈1) of AKI for eGFR >60 mL/min per 1.73 m2. The risk of AKI increased exponentially (Figure 3A) for lower eGFR values. Likewise, we found a similar, though softer, exponential relationship between baseline eGFR and 1‐year outcome (Figure 3B). Therefore, we included baseline eGFR as binary variable in the mediation analysis, using cutoffs at 30 (main analysis) and 45 mL/min per 1.73 m2 (sensitivity analysis), corresponding to CKD stage 4 to 5 and CKD stage 3b‐5, respectively.
Figure 3. Relationship between baseline eGFR and outcomes.

A, Relationship between baseline eGFR and AKI. Restricted cubic spline of the relationship between baseline eGFR and AKI, adjusted for median contrast dose of 181 mL. B, Relationship between baseline eGFR and all‐cause mortality 1‐year after TAVR. Restricted cubic spline of the relationship between baseline eGFR and all‐cause mortality one 1‐after TAVR. AKI indicates acute kidney injury; eGFR, estimated glomerular filtration rate; OR, odds ratio; and TAVR, transcatheter aortic valve replacement.
Mediation Analysis
As expected, baseline eGFR <30 mL/min per 1.73 m2 (CKD stage 4–5), bleeding complications and contrast dose were all significantly associated with the development of AKI (Table 4). CKD 4–5, AKI, bleedings, but not contrast dose except for the highest quartile (>250 mL), were significantly associated with the primary outcome (Figure 4).
Table 4.
Building Mediation Analysis
| ß | Exp (ß) | Lower 95% CI | Upper 95% CI | Pr (>|z|) | |
|---|---|---|---|---|---|
| Estimating direct effect on all‐cause at 1‐y follow up | |||||
| (Intercept) | −2.9846 | 0.05 | 0.03 | 0.07 | <0.001 |
| CKD 4–5—eGFR <30 (mL/min per 1.73 m2) | 1.2841 | 3.61 | 2.42 | 5.40 | <0.001 |
| Bleedings (BARC 2, 3, or 5) | 0.7427 | 2.10 | 1.51 | 2.93 | <0.001 |
| Contrast dose Q1 <137 mL [reference category] | |||||
| Q2 137–180 mL | −0.0271 | 0.97 | 0.59 | 1.59 | 0.9141 |
| Q3 181–250 mL | 0.2585 | 1.29 | 0.81 | 2.08 | 0.2851 |
| Q4 >250 mL | 0.7041 | 2.02 | 1.29 | 3.18 | 0.0023 |
| Estimating effect on mediator (AKI) | |||||
| (Intercept) | −2.622 | 0.07 | 0.05 | 0.10 | <0.001 |
| CKD 4–5—eGFR <30 (mL/min per 1.73 m2) | 1.537 | 4.65 | 3.32 | 6.52 | <0.001 |
| Bleedings (BARC 2, 3, or 5) | 0.441 | 1.55 | 1.19 | 2.03 | 0.0013 |
| Contrast dose Q1 <137 mL [reference category] | |||||
| Q2 137–180 mL | 0.578 | 1.78 | 1.20 | 2.65 | 0.0042 |
| Q3 181–250 mL | 0.918 | 2.50 | 1.70 | 3.68 | <0.001 |
| Q4 >250 mL | 1.144 | 3.14 | 2.13 | 4.62 | <0.001 |
| Estimating overall effect on all‐cause at 1‐y follow up | |||||
| (Intercept) | −3.0324 | 0.05 | 0.03 | 0.07 | <0.001 |
| CKD 4–5—eGFR <30 (mL/min per 1.73 m2) | 1.1176 | 3.06 | 2.01 | 4.64 | <0.001 |
| AKI | 0.6065 | 1.83 | 1.27 | 2.65 | 0.0012 |
| Bleedings (BARC 2, 3, or 5) | 0.7013 | 2.02 | 1.44 | 2.81 | <0.001 |
| Contrast dose Q1 <137 mL [reference category] | |||||
| Q2 137–180 mL | −0.0663 | 0.94 | 0.57 | 1.54 | 0.7931 |
| Q3 181–250 mL | 0.1907 | 1.21 | 0.75 | 1.95 | 0.4338 |
| Q4 >250 mL | 0.6099 | 1.84 | 1.16 | 2.91 | 0.0092 |
AKI indicates acute kidney injury; BARC, Bleeding Academic Research Consortium 20 ; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; and Q, quartile.
Figure 4. Disentanglement of baseline risk, TAVR associated complication, and clinical outcome.
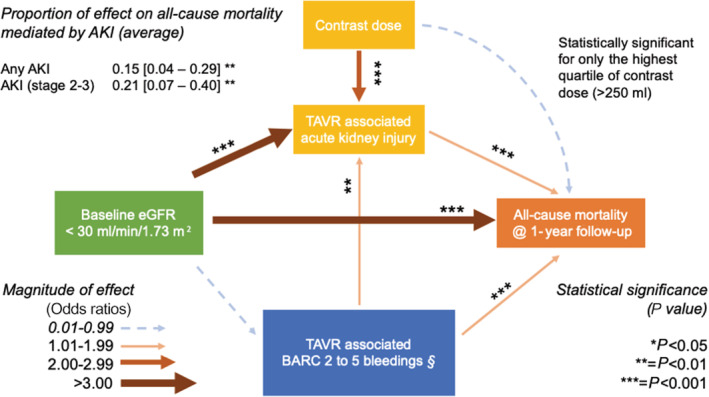
Arrows denote effect of independent variable on dependent variable. Contrast dose was not significantly associated with primary outcome except for the highest quartile. §Bleeding events assessed during hospitalization, excluding by study‐protocol, those occurring before 48 hours. AKI indicates acute kidney injury; BARC, Bleeding Academic Research Consortium; eGFR, estimated glomerular filtration rate; and TAVR, transcatheter aortic valve replacement.
The indirect effect of baseline eGFR on the primary outcome through AKI was characterized by an OR of 1.02 (95% CI, 1.01–1.07, P=0.0024) at bootstrap analysis. The estimated proportion of the total effect of eGFR <30 mL/min per 1.73 m2 (CKD stage 4–5) on the primary outcome mediated through AKI was on average 15% (95% CI, 4%–29%, P<0.001), being 10% (95% CI, 3%–22%, P<0.01) in patients who were non‐CKD 4 to 5 and 20% (95% CI, 6%–36%, P<0.01) in patients with CKD 4 to 5 patients, respectively (Tables 4 and 5).
Table 5.
Mediation Analysis of CKD Through AKI
| ß | Exp (ß) | 95% CI lower | 95% CI upper | P value | |
|---|---|---|---|---|---|
|
Any AKI CKD 4–5—eGFR <30 (mL/min per 1.73 m2) | |||||
| ACME (average) | 0.02276 | 1.02 | 0.00688 | 0.04 | 0.0024 |
| ADE (average) | 0.12788 | 1.14 | 0.06839 | 0.19 | <0.001 |
| Proportion of mediated (average) | 0.15107 | 0.04595 | 0.29 | 0.0024 | |
|
AKI stage 2 and 3 CKD 4–5—eGFR <30 (mL/min per 1.73 m2) | |||||
| ACME (average) | 0.03148 | 1.03 | 0.01086 | 0.05 | 0.0016 |
| ADE (average) | 0.12022 | 1.13 | 0.05968 | 0.18 | <0.001 |
| Proportion of mediated (average) | 0.20753 | 0.07058 | 0.4 | 0.0016 | |
|
Any AKI CKD 3–5—eGFR <30 (mL/min per 1.73 m2) | |||||
| ACME (average) | 0.01042 | 1.01 | 0.00317 | 0.02 | 0.0012 |
| ADE (average) | 0.08354 | 1.09 | 0.05065 | 0.12 | <0.001 |
| Proportion of mediated (average) | 0.11088 | 0.0334 | 0.2 | 0.0012 | |
ACME indicates average causal mediator effect; ADE, average direct effect; AKI, acute kidney injury; CKD, chronic kidney disease; and eGFR, estimated glomerular filtration rate.
Sensitivity Analyses
After excluding the 394 patients with any surgical access, the average mediation effect of AKI was still significant, being 11% (95% CI, 2%–24%, P<0.04) on the primary outcome.
Mediation analysis including only AKI stage 2 and 3 events showed an increased proportion of effect mediated by AKI, being 21% (95% CI, 7%–40%, P=0.0016) (Table 5).
When we used a less strict definition of CKD, setting a cutoff value at 45 mL/min per 1.73 m2 (CKD stage 3b‐5), the proportion of mediated effect by any AKI events was 11% (95% CI, 3%–20%, P=0.0012).
On the contrary, any BARC 2, 3, or 5 events were a nonsignificant mediator of CKD effect on the primary outcome, with an average proportion of mediated effect of 3% (95% CI, −2% to –11%, P<0.18) (Tables 4 and 5).
DISCUSSION
In this large contemporary cohort of patients undergoing TAVR in 5 high‐volume centers in Italy, we found that (1) AKI occurs in 1 out of 6 patients and doubled the risk of all‐cause mortality after 1‐year follow‐up, even after adjusting for baseline patient risk profile with either STS or logistic EuroSCORE II; and (2) baseline eGFR, bleeding complications, and iodinated contrast are predictors of primary outcome and AKI, with differential effects in a complex network of mutual interplay (shown in Figure 4). AKI, a potentially preventable complication, mediates one fifth of the effect of baseline CKD, one of the strongest predictors of mortality after TAVR, 9 , 10 , 11 on all‐cause mortality after 1‐year follow up.
AKI, variously defined, is a common complication after TAVR, with a widely variable incidence across studies ranging from 8.3% to 57% 4 , 10 , 25 , 26 , 27 and it is linked to increased short‐ and long‐term mortality. 28 In our cohort, AKI development was associated with a doubling of mortality risk after 1‐year follow‐up (15.9% versus 8.0%, P<0.001, Figure 2), with a clear stepwise effect: the worse the AKI stage, the worse the impact on mortality (Figures S1 through S4). This is in line with current literature 10 , 26 and, importantly, it was maintained after adjusting for universally accepted baseline risk stratifiers, supporting a strong link between AKI and unfavorable outcome after TAVR.
Nonetheless, baseline CKD and BARC bleedings resulted as both strong AKI and primary outcome predictors. Thus, whether AKI prevention may eventually translate into an improved clinical outcome is debatable. 8 , 14 , 22 To clarify this important point, we explored the interplay between baseline CKD; TAVR‐associated events such as AKI; BARC 2, 3, or 5 bleeds; and their association with 1‐year mortality. The disentanglement of these complex relationships is highlighted in Figure 4.
Patients with CKD stage 4 to 5 showed a significantly increased risk of AKI after TAVR. Both AKI and CKD independently affected 1‐year mortality, although the magnitude of effect of the latter was higher than that of the former. AKI mediated on average, one sixth to one fifth of the 1‐year mortality risk conferred by baseline CKD stage 4 to 5. This finding is of clear clinical relevance: indeed, 1 year after TAVR, the absolute risk difference between patients without and with CKD in our cohort is 8.7%, which leads to a theoretical number needed to treat of 11.5, if we could virtually improve eGFR and abate CKD. Nonetheless, our analysis shows that one fifth to one sixth of this effect can be tackled via AKI prevention, with a sizable number needed to treatranging around 50. We believe that this finding is truly hypothesis generating and might deserve to be tested in properly sized clinical trials.
Importantly, the results of mediation analysis were consistent among the explored subgroups; the effect of AKI was still significant in patients receiving fully percutaneous TAVR, which is relevant as surgical access has been reported as a strong predictor of AKI itself. 26 Furthermore, we found a gradient of effect with higher proportion of significant AKI‐mediated effect when we considered only severe (eg, stage 2–3) AKI. We found a lower proportion of AKI‐mediated effect when we included stage 3b in our CKD definition, giving further strength to the overall picture.
Being, to the best of our knowledge, the first mediation analysis on the role of AKI after TAVR, our result can be put into a perspective only with similar analyses in different scenarios. 22 Weisbord et al. looked at AKI in patients undergoing elective coronary procedures; even though they could ascertain an association between AKI and an increased incidence of clinical events (death, need for dialysis, or persistent impairment in kidney function at 90 days), the authors failed to show a significant proportion of effect mediated by AKI over the baseline risk conferred by CKD. The diverging results may be explained by different patients' characteristics (median age was 69 years, 93% men versus 82 years and 43% men in our cohort), different contrast dose (median 85 mL compared with >200 mL in our cohort), and, more likely, the different procedure. In fact, TAVR, as compared with percutaneous coronary intervention, is associated with a remarkable shift of the prerenal component responsible for SCr change, being characterized by a transient hypotension during valve implantation, rapidly followed by an increase in cardiac and urine output owinge to the acutely reduced afterload. 29 , 30 , 31
Our results, instead, are consistent with those shown by Rothenbühler et al. in a mediation post hoc analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, 14 in which bleeding events were taken into account. The authors showed that AKI and bleedings were mutually linked and that the mortality benefit of the radial as compared with the femoral approach was mainly driven by AKI and not by the well‐known reduction in bleeding rates afforded by radial access. This is in line with the disentanglement of the risks of TAVR‐associated complications, with the highlighting of AKI as an independent player (see Figure 4), and with the lack of significant mediator effect of bleeding events seen in our analysis.
Regarding contrast reduction 8 largely considered as the mainstay of AKI prevention, any increase in dye dose compared with the lowest quartile significantly raised the risk of AKI (Table 5), whereas only the highest contrast quartile was significantly associated with the primary outcome. In this view, it is likely that contrast dose mainly acts as proxy for complex or complicated TAVR procedures. In addition, even after adjusting for contrast dose the risk of developing AKI was very low for a baseline eGFR above 45 mL/min per 1.73 m2, (see Figure 3A), setting a reasonable cutoff for patients deserving heightened attention and stronger AKI prevention efforts.
Limitations
The first limitation to acknowledge is the retrospective nature of analysis; nonetheless, data derive from prospectively collected local registries of 5 high‐volume TAVR centers. This could partially explain the relatively high use of contrast medium in comparison to other large randomized prospective studies.
Second, SCr assessment and need for hemodialysis after discharge were not routinely recorded, and therefore we could not include them as outcome measures. Third, the timing of in‐hospital bleeding events was not consistently collected, negating multistate and competing risk models. 14 However, multiple sensitivity analysis showed consistent results with a clear gradient in associated risk between CKD stages; moreover, the severity of AKI was proportional to the percentage of mediated effect. Finally, we could not find a significant association between CKD and in‐hospital BARC 2, 3, and 5 bleedings. Whereas this was not a focal point of our analysis, this finding may derive by the exclusion of earlier events after TAVR by study protocol and is in line with the result of a recent meta‐analysis. 10
AKI occurred in 1 out of 6 patients after TAVR and doubled the risk of all‐cause mortality after 1‐year follow‐up, even after adjusting for baseline risk profile. AKI mediates one fifth of the effect of baseline CKD on all‐cause mortality. Our analysis supports a systematic effort to prevent AKI after TAVR by reducing contrast dose and minimizing bleeding complications.
CONCLUSIONS
Among a large cohort of patients undergoing TAVR, we demonstrated that AKI occurs in one sixth and significantly mediates one fifth of the effect of baseline CKD on all‐cause mortality after the procedure. Further studies are urgently needed to disentangle this complex scenario and to encourage a systematic effort to prevent AKI during TAVR, finally potentially leading to an improvement of patients' 1‐year survival.
Sources of Funding
None.
Disclosures
Dr Crimi reports speaker fees in the past 2 years from Abbott, Astra Zeneca, Bayer Boehringer‐Ingelheim, and Daiichi‐Sankyo, not related to this work. Dr Morici reports lecture fees Pfizer/Bristol‐Myers Squibb and grant research from Getinge Global USA, outside of the submitted work. Dr Esposito has been supported by a research grant provided by the Cardiopath PhD program, not specifically related to this work. Professor De Ferrari reports consultant or speaker fees in the past 2 years from Sanofi, Amgen, and UCB, not related to this work. Dr Vercellino has received speaker fees from Sanofi, Shockwave Medical Inc, Bristol‐Myers Squibb, and Bayer not related to this work. Dr Bedogni is a consultant and proctor for Medtronic, Abbott, BSCI, Meril, and Terumo. Professor Porto reports consultant or speaker fees in the past 2 years from Biotronik, ABIOMED, Terumo, Philips, Sanofi, Amgen, Daiichi‐Sankyo, Astra Zeneca, and Bayer, not related to this work. The coauthors not mentioned here declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Figures S1–S4
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024589
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Baumgartner H, Falk V, Bax JJ, de Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2786. doi: 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 2. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e229. doi: 10.1161/CIR.0000000000000955 [DOI] [PubMed] [Google Scholar]
- 3. Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, Piazza N. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014;107:133–139. doi: 10.1016/j.acvd.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 4. Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, Frey FJ. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–2179. doi: 10.1093/ndt/gfp036 [DOI] [PubMed] [Google Scholar]
- 5. Moriyama N, Laakso T, Raivio P, Dahlbacka S, Kinnunen EM, Juvonen T, Valtola A, Husso A, Jalava MP, Ahvenvaara T, et al. Acute kidney injury following aortic valve replacement in patients without chronic kidney disease. Can J Cardiol. 2021;37:37–46. doi: 10.1016/j.cjca.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto M, Hayashida K, Mouillet G, Chevalier B, Meguro K, Watanabe Y, Dubois‐Rande JL, Morice MC, Lefèvre T, Teiger E. Renal function‐based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:479–486. doi: 10.1016/j.jcin.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 7. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath‐PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehran R, Dangas GD, Weisbord SD. Contrast‐associated acute kidney injury. N Engl J Med. 2019;380:2146–2155. doi: 10.1056/NEJMra1805256 [DOI] [PubMed] [Google Scholar]
- 9. Voigtländer L, Schewel J, Martin J, Schewel D, Frerker C, Wohlmuth P, Thielsen T, Kuck KH, Schäfer U. Impact of kidney function on mortality after transcatheter valve implantation in patients with severe aortic valvular stenosis. Int J Cardiol. 2015;178:275–281. doi: 10.1016/j.ijcard.2014.10.172 [DOI] [PubMed] [Google Scholar]
- 10. Gargiulo G, Capodanno D, Sannino A, Perrino C, Capranzano P, Stabile E, Trimarco B, Tamburino C, Esposito G. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta‐analysis of 4992 patients. Circ Cardiovasc Interv. 2015;8:e002220. doi: 10.1161/CIRCINTERVENTIONS.114.002220 [DOI] [PubMed] [Google Scholar]
- 11. Gupta T, Goel K, Kolte D, Khera S, Villablanca PA, Aronow WS, Bortnick AE, Slovut DP, Taub CC, Kizer JR, et al. Association of chronic kidney disease with in‐hospital outcomes of transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10:2050–2060. doi: 10.1016/j.jcin.2017.07.044 [DOI] [PubMed] [Google Scholar]
- 12. Chandrasekhar J, Sartori S, Mehran R, Aquino M, Vogel B, Asgar AW, Webb JG, Tchetche D, Dumonteil N, Colombo A, et al. Incidence, predictors, and outcomes associated with acute kidney injury in patients undergoing transcatheter aortic valve replacement: from the BRAVO‐3 randomized trial. Clin Res Cardiol. 2021;110:649–657. doi: 10.1007/s00392-020-01787-7 [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Yu W, Zhou Y, Yang Y, Li C, Liu N, Hou X, Wang L. Independent risk factors contributing to acute kidney injury according to updated valve academic research consortium‐2 criteria after transcatheter aortic valve implantation: a meta‐analysis and meta‐regression of 13 studies. J Cardiothorac Vasc Anesth. 2017;31:816–826. doi: 10.1053/j.jvca.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 14. Rothenbühler M, Valgimigli M, Odutayo A, Frigoli E, Leonardi S, Vranckx P, Turturo M, Moretti L, Amico F, Uguccioni L, et al. Association of acute kidney injury and bleeding events with mortality after radial or femoral access in patients with acute coronary syndrome undergoing invasivemanagement: secondary analysis of a randomized clinical trial. Eur Heart J. 2019;40:1226–1232. doi: 10.1093/eurheartj/ehy860 [DOI] [PubMed] [Google Scholar]
- 15. Gilard M, Eltchaninoff H, Iung B, Donzeau‐Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, et al. Registry of transcatheter aortic‐valve implantation in high‐risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705 [DOI] [PubMed] [Google Scholar]
- 16. Tamburino C, Barbanti M, D'Errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, et al. 1‐year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT study. J Am Coll Cardiol. 2015;66:804–812. doi: 10.1016/j.jacc.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 18. Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, et al. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17:204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–1857. doi: 10.1093/eurheartj/ehaa799 [DOI] [PubMed] [Google Scholar]
- 20. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 21. Imai K, Keele L, Tingley D, Yamamoto T. Causal Mediation Analysis Using R. SpringerLink; 2010:129–154. [Google Scholar]
- 22. Weisbord SD, Palevsky PM, Kaufman JS, Wu H, Androsenko M, Ferguson RE, Parikh CR, Bhatt DL, Gallagher M. Contrast‐associated acute kidney injury and serious adverse outcomes following angiography. J Am Coll Cardiol. 2020;75:1311–1320. doi: 10.1016/j.jacc.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 23. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 24. Tibshirani RJ, Efron B. An Introduction to the Bootstrap. Chapman and Hall; 1993. [Google Scholar]
- 25. Barbash IM, Ben‐Dor I, Dvir D, Maluenda G, Xue Z, Torguson R, Satler LF, Pichard AD, Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. doi: 10.1016/j.ahj.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 26. Liao YB, Deng XX, Meng Y, Zhao ZG, Xiong TY, Meng XJ, Zuo ZL, Li YJ, Cao JY, Xu YN, et al. Predictors and outcome of acute kidney injury after transcatheter aortic valve implantation: a systematic review and meta‐analysis. EuroIntervention. 2017;12:2067–2074. doi: 10.4244/EIJ-D-15-00254 [DOI] [PubMed] [Google Scholar]
- 27. Saia F, Ciuca C, Taglieri N, Marrozzini C, Savini C, Bordoni B, Dall'Ara G, Moretti C, Pilato E, Martn‐Suàrez S, et al. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol. 2013;168:1034–1040. doi: 10.1016/j.ijcard.2012.10.029 [DOI] [PubMed] [Google Scholar]
- 28. De Marzo V, Crimi G, Benenati S, Buscaglia A, Pescetelli F, Vercellino M, Della Bona R, Sarocchi M, Canepa M, Ameri P, et al. BMI and acute kidney injury post transcatheter aortic valve replacement: unveiling the obesity paradox. J Cardiovasc Med (Hagerstown). 2021;22:579–585. doi: 10.2459/JCM.0000000000001178 [DOI] [PubMed] [Google Scholar]
- 29. Lemes da Silva MV, Nunes Filho ACB, Rosa VEE, Caixeta A, Lemos Neto PA, Ribeiro HB, Almeida BO, Mariani J, Campos CM, Abizaid AAC, et al. Improvement of renal function after transcatheter aortic valve replacement in patients with chronic kidney disease. PLoS One. 2021;16:e0251066. doi: 10.1371/journal.pone.0251066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azarbal A, Malenka DJ, Huang Y‐L, Ross CS, Solomon RJ, DeVries JT, Flynn JM, Butzel D, McKay M, Dauerman HL. Recovery of kidney dysfunction after transcatheter aortic valve implantation (from the Northern New England Cardiovascular Disease Study Group). Am J Cardiol. 2019;123:426–433. doi: 10.1016/j.amjcard.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 31. Venturi G, Pighi M, Pesarini G, Ferrero V, Lunardi M, Castaldi G, Setti M, Benini A, Scarsini R, Ribichini FL. Contrast‐induced acute kidney injury in patients undergoing TAVI compared with coronary interventions. J Am Heart Assoc. 2020;9:e017194. doi: 10.1161/JAHA.120.017194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S4


