Clonal hematopoiesis is a hallmark of aging, and its associations with cardiovascular diseases have been documented in multiple human cohorts. 1 Heart failure is a leading cause of death, particularly in older adults, and its relationship with clonal hematopoiesis has been elaborated by a series of epidemiological and experimental studies. Recently, Yu et al reported that incident heart failure is associated with clonal hematopoiesis in an analysis of 56 597 individuals from 5 study cohorts. 2 It was found that prevalent somatic mutation variants of TET2, JAK2, and ASXL1, commonly referred to as clonal hematopoiesis “driver” genes, are individually associated with an increased risk of developing heart failure. Furthermore, subgroup analysis revealed that only mutations in ASXL1 were associated with reduced left ventricular ejection fraction. While prior experimental studies have provided mechanistic evidence for causal relationships between various clonal hematopoiesis driver genes and heart failure, 1 the relationship between left ventricular dysfunction and variants in ASXL1 has not been previously evaluated by mechanistic experiments.
The authors will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedures used in this publication. Additional supporting data are available from the corresponding author upon reasonable request. All procedures involving animal subjects have been approved by the Institutional Animal Care and Use Committee at the University of Virginia under Walsh protocol 4205. Mice heterozygous for the knock‐in Asxl1 p.G643WfsX12 (Asxl1 tm/+ ) mutation, which gives rise to a truncated protein, were used in these studies because the mutation is physiologically expressed by its native promoter and this model mimics the most frequently detected mutations in the human ASXL1 driver gene (Figure A). 3
Figure 1. Experimental system and consequences of hematopoietic Asxl1 mutagenesis.
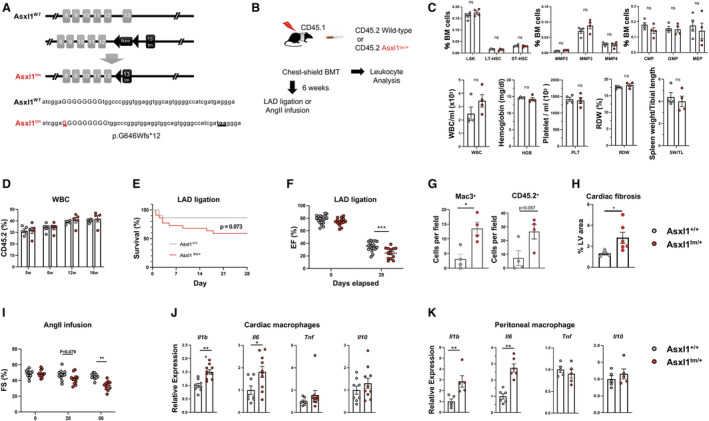
A, Schematic of the knock‐in Asxl1 allele. The insertion of a guanine nucleotide generates a premature stop codon resulting in a truncated protein at exon 13. B, Schematic of the experimental design using chest‐shielded BMT. B6 CD45.1 Pep Boy recipient animals were irradiated with 2 radiation doses of 5.5 Gy 4 h apart using a lead shield to protect the heart from radiation injury. Mice received 4×106 (for chimerism analysis) or 8×106 (for experimental heart failure models) bone marrow cells from C57BL/6J wild‐type or Asxl1 tm/+ mice. C, Hematopoietic cell parameters and spleen weights of mice transplanted with Asxl1 tm/+ and littermate wild‐type bone marrow at 8 weeks post‐BMT. Cells were analyzed by flow cytometry and an Element HT5 Veterinary Hematology Analyzer. 4 Statistical significance was evaluated by multiple Student t test (hematopoietic stem and progenitor cells) or by 2‐tailed unpaired Student t test (others). D, The chimerism of transplanted CD45.2 cells was analyzed by flow cytometry at indicated time points (n=5 per genotype). Statistical significance was evaluated by a 2‐way repeated‐measures ANOVA. E and F, 6 weeks after chest‐shielded BMT, LAD ligation was performed. E, The Kaplan–Meier curve shows survival after LAD ligation. The mortality rates of the wild‐type mice and Asxl1 tm/+ mice after surgery were 13.6% and 41.0%, respectively, during the follow‐up period. An exact log‐rank test was used for statistical analysis. F, Echocardiographic evaluation at 28 days after LAD artery ligation on surviving mice to assess ejection fraction (n=19 for wild‐type and n=13 for Asxl1 tm/+ , respectively). Ejection fraction was calculated from parasternal long‐axis view in VevoLab, which utilizes modified Simpson's biplane method of disk summation. The statistical test was 2‐way repeated‐measures ANOVA. G, Masson’s trichrome staining quantification of left ventricle fibrosis area. (n=4 for wild‐type and n=6 for Asxl1 tm/+ , respectively; mean±SEM; Mann–Whitney U test). H, Quantification of positive MAC3+ macrophages and CD45.2 cells in heart after LAD ligation by immunofluorescence staining of paraffin sections (n=4 mice; 10 fields of view per mouse; mean±SEM; *P<0.05, Mann–Whitney U test). I, Sequential analysis of echocardiographic analysis shows the effect of Asxl1 tm/+ BMT on fractional shortening before the infusion (Day 0) of angiotensin II (2.0 mg/kg per day) (n=11 for wild‐type and n=12 for Asxl1 tm/+ ), and after 28 and 58 days. The statistical test was by 2‐way repeated‐measures ANOVA. J, Cytokine transcript expression by cardiac macrophages isolated from mice transplanted with wild‐type or Asxl1 tm/+ bone marrow. CD64+ macrophages were sorted by flow cytometry after 58 days of angiotensin II infusion. K, Cytokine expression by peritoneal macrophages isolated from wild‐type and Asxl1 tm/+ mice. Elicited peritoneal macrophages were stimulated with 10 ng/mL lipopolysaccharide and 2 ng/mL interferon‐γ for 6 h and transcript expression was analyzed by quantitative reverse transcription polymerase chain reaction. Statistical significance was evaluated by 2‐tailed unpaired Student t tests. BMT indicates bone marrow transplantation; CMP, common myeloid progenitor; EF, ejection fraction; FS, fractional shortening; GMP, granulocyte‐monocyte progenitor; HGB, hemoglobin; LSK, Lin−Sca‐1+c‐Kit+ cells; LT‐HSC, long‐term hematopoietic stem cells; MEP, megakaryocyte‐erythrocyte progenitor; MMP, multipotent progenitors; ns, not significant; PLT, platelet; RDW, red blood cell distribution width; ST‐HSC, short‐term hematopoietic stem cells; SW, spleen weight; TL, tibial length; tm, truncating mutation; WBC, white blood cells; and WT, wild‐type. *P<0.05; **P<0.01; ***P<0.001.
To test the hypothesis that ASXL1‐mediated clonal hematopoiesis contributes to heart failure and left ventricular dysfunction, we established a murine model using chest‐shielded bone marrow transplantation (BMT) to eliminate the potential effects of radiation‐induced cardiac tissue damage (Figure B). After irradiation, recipient mice were transplanted with bone marrow cells from either Asxl1 tm/+ or littermate wild‐type mice, and hematopoietic and cardiac parameters were assessed as described previously. 4 Consistent with the clinical paradigm of clonal hematopoiesis, BMT with Asxl1 tm/+ donor cells did not lead to alterations in levels of hematopoietic stem and progenitor cells subpopulations, white blood cells, hemoglobin, or platelets, and did not affect red blood cell distribution width or spleen weight (Figure C). Consistent with previous studies, 3 the BMT of CD45.2, Asxl1 tm/+ donor cells did not show competitive expansion in white blood cells compared with wild‐type (Figure D). Donor cell engraftment was 52.5±9.8% in white blood cells at 6 weeks after BMT, representing a mutant allele fraction of 26% that is within the range observed in clonal hematopoiesis carriers. Following left anterior descending (LAD) artery ligation, mice showed a trend toward greater mortality in the Asxl1 tm/+ group compared with the control group (Figure E). Echocardiographic analysis of surviving animals showed that, at 28 days after LAD artery ligation, the group that underwent Asxl1 tm/+ BMT displayed significantly lower left ventricular ejection fraction compared with the control group (Figure F), and this was accompanied by the infiltration of CD45.2 leukocytes, greater macrophage infiltration, and more reactive fibrosis of the left ventricle (Figure G and H). To extend these results to a model of nonischemic heart failure, a separate set of control and test mice were infused with a supraphysiological dose of angiotensin II to induce cardiac damage from persistent, uncontrolled hypertension. The Asxl1 tm/+ ‐transplanted group of mice displayed greater left ventricular systolic dysfunction by 56 days and a trend toward greater cardiac dysfunction by 28 days (Figure I). Collectively, these findings provide evidence for a causal relationship between ASXL1‐mediated clonal hematopoiesis and heart failure.
Many clonal hematopoiesis driver gene candidates are believed to promote heart failure through pro‐inflammatory mechanisms involving inflammasome activation and cytokine overproduction by myeloid cells. 1 Thus, we tested whether the Asxl1 variant could also confer a pro‐inflammatory phenotype to macrophages. At the termination of the AngII infusion experiment, cardiac macrophages isolated from mice transplanted with Asxl1tm/+ bone marrow expressed higher levels of the transcripts that encode for IL‐1β and IL‐6 among a group of representative cytokines involved in cardiac inflammation (Figure J). Similarly, peritoneal macrophages harvested from Asxl1 tm/+ mice and littermate wild‐type animals expressed higher levels of Il1b and Il6 following incubation with lipopolysaccharide and interferon‐γ (Figure K), consistent with previous findings on TET2 variants and other clonal hematopoiesis driver genes. 1 , 4 , 5 Because the link between IL‐1β and IL‐6 cytokines and prognosis in patients with heart failure has been well documented, 1 these data support a mechanism of accelerated heart failure caused by elevated cytokine production that results from ASXL1‐mediated clonal hematopoiesis.
Sources of Funding
This work was funded by National Institutes of Health (Bethesda, MD) grants AG073249, HL141256, HL152174, and HL139819. There are no relationships with industry to report.
Disclosures
None.
Acknowledgments
The Asxl1 pG643WfsX12 mouse strain was kindly provided by Dr Wen‐Chien Chou in National Taiwan University College of Medicine. We thank Heather Doviak for assistance with these experiments.
For Sources of Funding and Disclosures, see page 3.
References
- 1. Min KD, Kour A, Sano S, Walsh K. The role of clonal haematopoiesis in cardiovascular diseases: epidemiology and experimental studies. J Intern Med. 2020;288:507–517. doi: 10.1111/joim.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu B, Roberts MB, Raffield LM, Zekavat SM, Nguyen NQH, Biggs ML, Brown MR, Griffin G, Desai P, Correa A, et al; National Heart, Lung, and Blood Institute TOPMed Consortium . Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol. 2021;78:42–52. doi: 10.1016/j.jacc.2021.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsu YC, Chiu YC, Lin CC, Kuo YY, Hou HA, Tzeng YS, Kao CJ, Chuang PH, Tseng MH, Hsiao TH, et al.The distinct biological implications of Asxl1 mutation and its roles in leukemogenesis revealed by a knock‐in mouse model. J Hematol Oncol. 2017;10:139. doi: 10.1186/s13045-017-0508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, et al. Tet2‐mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL‐1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381 [DOI] [PMC free article] [PubMed] [Google Scholar]


