Figure 1. Experimental system and consequences of hematopoietic Asxl1 mutagenesis.
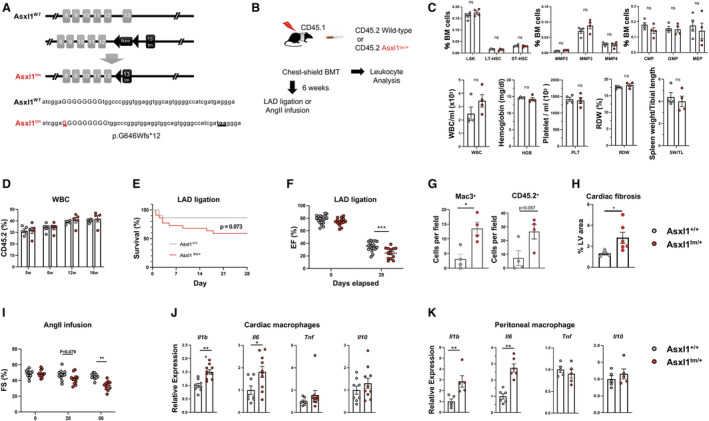
A, Schematic of the knock‐in Asxl1 allele. The insertion of a guanine nucleotide generates a premature stop codon resulting in a truncated protein at exon 13. B, Schematic of the experimental design using chest‐shielded BMT. B6 CD45.1 Pep Boy recipient animals were irradiated with 2 radiation doses of 5.5 Gy 4 h apart using a lead shield to protect the heart from radiation injury. Mice received 4×106 (for chimerism analysis) or 8×106 (for experimental heart failure models) bone marrow cells from C57BL/6J wild‐type or Asxl1 tm/+ mice. C, Hematopoietic cell parameters and spleen weights of mice transplanted with Asxl1 tm/+ and littermate wild‐type bone marrow at 8 weeks post‐BMT. Cells were analyzed by flow cytometry and an Element HT5 Veterinary Hematology Analyzer. 4 Statistical significance was evaluated by multiple Student t test (hematopoietic stem and progenitor cells) or by 2‐tailed unpaired Student t test (others). D, The chimerism of transplanted CD45.2 cells was analyzed by flow cytometry at indicated time points (n=5 per genotype). Statistical significance was evaluated by a 2‐way repeated‐measures ANOVA. E and F, 6 weeks after chest‐shielded BMT, LAD ligation was performed. E, The Kaplan–Meier curve shows survival after LAD ligation. The mortality rates of the wild‐type mice and Asxl1 tm/+ mice after surgery were 13.6% and 41.0%, respectively, during the follow‐up period. An exact log‐rank test was used for statistical analysis. F, Echocardiographic evaluation at 28 days after LAD artery ligation on surviving mice to assess ejection fraction (n=19 for wild‐type and n=13 for Asxl1 tm/+ , respectively). Ejection fraction was calculated from parasternal long‐axis view in VevoLab, which utilizes modified Simpson's biplane method of disk summation. The statistical test was 2‐way repeated‐measures ANOVA. G, Masson’s trichrome staining quantification of left ventricle fibrosis area. (n=4 for wild‐type and n=6 for Asxl1 tm/+ , respectively; mean±SEM; Mann–Whitney U test). H, Quantification of positive MAC3+ macrophages and CD45.2 cells in heart after LAD ligation by immunofluorescence staining of paraffin sections (n=4 mice; 10 fields of view per mouse; mean±SEM; *P<0.05, Mann–Whitney U test). I, Sequential analysis of echocardiographic analysis shows the effect of Asxl1 tm/+ BMT on fractional shortening before the infusion (Day 0) of angiotensin II (2.0 mg/kg per day) (n=11 for wild‐type and n=12 for Asxl1 tm/+ ), and after 28 and 58 days. The statistical test was by 2‐way repeated‐measures ANOVA. J, Cytokine transcript expression by cardiac macrophages isolated from mice transplanted with wild‐type or Asxl1 tm/+ bone marrow. CD64+ macrophages were sorted by flow cytometry after 58 days of angiotensin II infusion. K, Cytokine expression by peritoneal macrophages isolated from wild‐type and Asxl1 tm/+ mice. Elicited peritoneal macrophages were stimulated with 10 ng/mL lipopolysaccharide and 2 ng/mL interferon‐γ for 6 h and transcript expression was analyzed by quantitative reverse transcription polymerase chain reaction. Statistical significance was evaluated by 2‐tailed unpaired Student t tests. BMT indicates bone marrow transplantation; CMP, common myeloid progenitor; EF, ejection fraction; FS, fractional shortening; GMP, granulocyte‐monocyte progenitor; HGB, hemoglobin; LSK, Lin−Sca‐1+c‐Kit+ cells; LT‐HSC, long‐term hematopoietic stem cells; MEP, megakaryocyte‐erythrocyte progenitor; MMP, multipotent progenitors; ns, not significant; PLT, platelet; RDW, red blood cell distribution width; ST‐HSC, short‐term hematopoietic stem cells; SW, spleen weight; TL, tibial length; tm, truncating mutation; WBC, white blood cells; and WT, wild‐type. *P<0.05; **P<0.01; ***P<0.001.
