Abstract
Purpose
Our study aims to assess the role and diagnostic performance of 3 different contrast-enhanced, abbreviated magnetic resonance imaging (MRI) protocols as a screening tool of hepatocellular carcinoma (HCC).
Material and methods
Our retrospective study included 80 patients who were screened for HCC: 47 patients revealed 138 focal hepatic lesions. MRI examinations were performed including full CE-MRI protocols. The MRI was done on a 1.5 T machine. Then 3 different abbreviated contrast-enhanced MRI protocols were analysed separately. The standard dynamic contrast MRI and abbreviated protocols were evaluated following the LI-RADS 2018 lexicon diagnostic features.
Results
A considerable overall kappa (k) agreement between the abbreviated 1, 2, and 3 protocols on LI-RADS classification was noted with k = 0.865. There was almost perfect agreement between all abbreviated protocols and full standard protocol on LI-RADS classification, with k = 0.890. As regards the k agreement on LI-RADS classification, there was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol, with k = 0.980. The abbreviated 1 and 2 protocols showed high diagnostic performance on LI-RADS classification of lesions, with 100% sensitivity, specificity, PPV, NPV, and accuracy, while the abbreviated 3 protocol showed a lesser but comparable sensitivity 96.9%, NPV 99.4, and accuracy 99.4%.
Conclusions
Abbreviated contrast-enhanced MRI protocols can be used as a screening tool for the detection of HCC, with high sensitivity, specificity, PPV, NPV, and accuracy close to the full protocol. There was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol. Subsequently, this protocol can be used as a standard protocol for screening high-risk patients.
Keywords: abbreviated, HCC, surveillance, magnetic resonance imaging, dynamic contrast
Introduction
Screening for hepatocellular carcinoma (HCC) is basic for early detection and characterization in high-risk patients. It is highly recommended by universal guidelines as a standard practice. However, using ultrasound as a usual tool in surveillance has many limitations, especially in high-risk patients with problematic or non-diagnostic checks [1].
The American Association for the Diagnosis of Liver Diseases prescribes semi-annual observation with ultrasound (US) and assurance of the α-fetoprotein level. Even though this methodology appears to diminish mortality from HCC by 37%, numerous centres adopt full contrast-enhanced magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) due to their more noteworthy affectability, compared with that of US. Nevertheless, the high cost and long duration of MRI limits its scheduled utility [2].
Due to the non-satisfactory execution of the current screening conventions, there is a requirement for a more sensitive and diagnostic screening technique. Abbreviated MRI has been assessed for screening of HCC in high-risk individuals. Abbreviated MRI offers points of interest such as time and cost-effectiveness over the full standard MRI protocol [3].
Hepatic MRI may take 30-40 within the regular full standard protocol. However, with an abbreviated MRI that includes only one series of imaging followed by post-contrast T1-weighted sequences, all the data required to distinguish liver injuries and assign suitable LI-RADS classification can be obtained. These abbreviated MRI examinations would probably only take 7-10 minutes, thus saving time and at a lower cost than standard full MRI [4].
Our study aims to assess the role and diagnostic performance of 3 different contrast-enhanced abbreviated MRI protocols as a screening tool of HCC in patients with chronic hepatitis C virus (HCV) infection.
Material and methods
This retrospective study was approved by the Ethics Review Board of our institution. Eighty patients with chronic HCV infection were screened. Forty-seven patients revealed 138 focal hepatic lesions. Thirty-three patients no revealed no focal lesions. The study was conducted from August 2021 to December 2021. The method was clarified in full, and informed consent was obtained from the participants. Our study included patients with chronic HCV. Other patients with other causes of liver cirrhosis were excluded from the study. Patients with contraindications for MRI (electronic pacemakers, MRI-incompatible implanted devices, intole-rant patients), patients with previous locoregional therapy for focal lesions, and patients with contraindications to MRI contrast media were also excluded from the study.
Clinical and laboratory data were precisely reviewed and included viral markers, HCV-PCR, liver function tests, and serum alpha-fetoprotein. MRI examinations were performed including full CE-MRI protocols. The MRI was done on a 1.5 T machine (Philips Achieva), using a 16-channel phased-array torso coil involving the whole abdominal length. The position of the patients was supine, headfirst on the table, then the contrast-enhanced full MRI protocol was obtained including axial T2-WI with single-shot TSE technique (1 min 48 sec), axial turbo spin-echo (TSE) fat-suppressed T2-WI (SPIR) (1 min 48 sec), axial heavy SSH/he T2WI (1 min 48 sec) and axial DWI series (2 min), obtained using 3 different b values: 0, 200, and 800 (TR 1300 MS, TE 64 MS), with associated ADC map, axial GRE T1-weighted out-phase/in-phase axial images (1 min 10 sec). CE-MRI was performed afterward using gadopentetate dimeglumine (Omnivist®) infused at a rate of 3 ml/min over 15 s at a dose of 0.2 ml/kg. It was followed by a saline chaser of 30–40 ml at a rate of 1-2 ml/s. Dynamic contrast-enhanced sequences were acquired using T1W high-resolution isotropic volume examination (THRIVE) obtained before (pre-contrast) and after contrast injection at 15, 20 (arterial phase), 40 (portal phase), 60 (venous phase), and 180 s (delayed phase). The involved contrast sequences were obtained at the usual axial plane (7 min). Hence, the total duration of the standard protocol was at least 18 minutes.
Then abbreviated MRI protocols were analysed separately and included abbreviated 1 protocol, axial SSTSE T2-weighted (1 min 48 sec), and axial contrast-enhanced T1-weighted with fat saturation (arterial, portal, and delayed phases) (7 min). The total duration of this abbreviated protocol is 8 min 48 sec. The abbreviated 2 MRI protocol was as follows: Axial diffusion-weighted (2 min) with associated ADC, and axial contrast-enhanced T1-weighted with fat saturation (arterial, portal, and delayed phases) (7 min). The total duration of this abbreviated protocol is 9 min. The abbreviated 3 MRI protocol was as follows: axial GRE T1-weighted out-phase/in-phase axial images (1 min 10 sec). Axial contrast-enhanced T1-weightedwith fat saturation (arterial, portal, and delayed phases) (7 min). The total duration of this abbreviated protocol is 8 min 10 sec.
The standard dynamic contrast MRI and abbreviated protocols were evaluated following the LI-RADS 2018 lexicon diagnostic features. The full protocol was analysed by a head consultant radiologist with 10 years of experience in body MRI. Afterward, 2 different consultant radiologists with 6 years of experience in body MRI analysed each abbreviated technique (with at least 8 weeks’ gap between each analysis of individual sets of images) separately and independently without knowing the results of the full protocol, to avoid bias. In case of mismatching results between 2 radiologists in some cases of abbreviated protocols, the head consultant radiologist revised their result to determine the final result of each abbreviated set of images. The observers were blinded to clinical MRI reports and/or any pathologic results. The observers randomly analysed 2 sets of images in 2 different sessions separated by at least 8 weeks to decrease recall bias. Our gold standard for evaluation of abbreviated protocols in screening HCC was the full standard protocol.
Statistical analysis
The data were analysed using Statistical Package for the Social Sciences (IBM Corp., released 2013; IBM SPSS Statistics for Windows, V. 22.0; Armonk, NY, USA). Qualitative data are described using numbers and percentages. Parametric quantitative data were expressed as mean ± standard deviation (SD). The diagnostic performance including the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the categorical variable was performed using cross-tabulation. Fleiss k was used to measure the inter-method agreement between 3 methods, and Cohen’s k coefficient (κ) was calculated to test the inter-method agreement between 2 methods. The 95% confidence interval was calculated. The k test was statistically significant when the p-value was less than 0.05. Kappa agreement was interpreted as follows: 0.01-0.20: slight agreement; 0.21-0.40: fair agreement; 0.41-0.60: moderate agreement; 0.61-0.80: substantial agreement; and 0.81-0.99: almost perfect agreement.
Results
Eighty patients were screened, 47 patients revealed 138 focal hepatic lesions. No focal lesions were detected on 33 patients. There were 49 females (28.7%) and 31 males (18.1%). The mean patient age was 52.8 ± 13.36 years (range 23 to 75 years). The mean size of lesions was 2.8 ± 2.2 cm (range 0.5 to 16.2 cm).
According to the full standard reference protocol, 34% of lesions were classified as LI-RADS 1, 6.5% of lesions as LIRADS2, 1.4% of lesions as LI-RADS 3, 0.72% of lesions as LI-RADS 4, 13.77% of lesions as LI-RADS 5 (Figure 1), 8.7% lesions as LI-RADS TIV (Figure 2), and 34.06% of lesions as LI-RADS M (Figure 3 and Table 1). However, the abbreviated 1 protocol showed that 34.78% lesions were classified as LI-RADS 1, 5.8% of lesions as LIRADS 2, and 2.1% of lesions as LI-RADS 3; while LI-RADS 4, LI-RADS 5, LI-RADS TIV, and LI-RADS M showed the same percentages (Table 2). According to the abbreviated 2 protocol 34% of lesions were classified as LI-RADS 1, 4.3% of lesions as LI-RADS 2, and 4.3% of lesions as LI-RADS 3, while LI-RADS 4, LI-RADS 5, LI-RADS TIV, and LI-RADS M showed the same percentages (Table 3). On the other hand, the abbreviated 3 protocol revealed that 25.3% of lesions were classified as LI-RADS 1, 12.3% were LI-RADS 3 (Figure 4), and no lesions were LI-RADS 4, while LI-RADS 2, LI-RADS 5, LI-RADS TIV, and LI-RADS M showed the same percentages (Table 4).
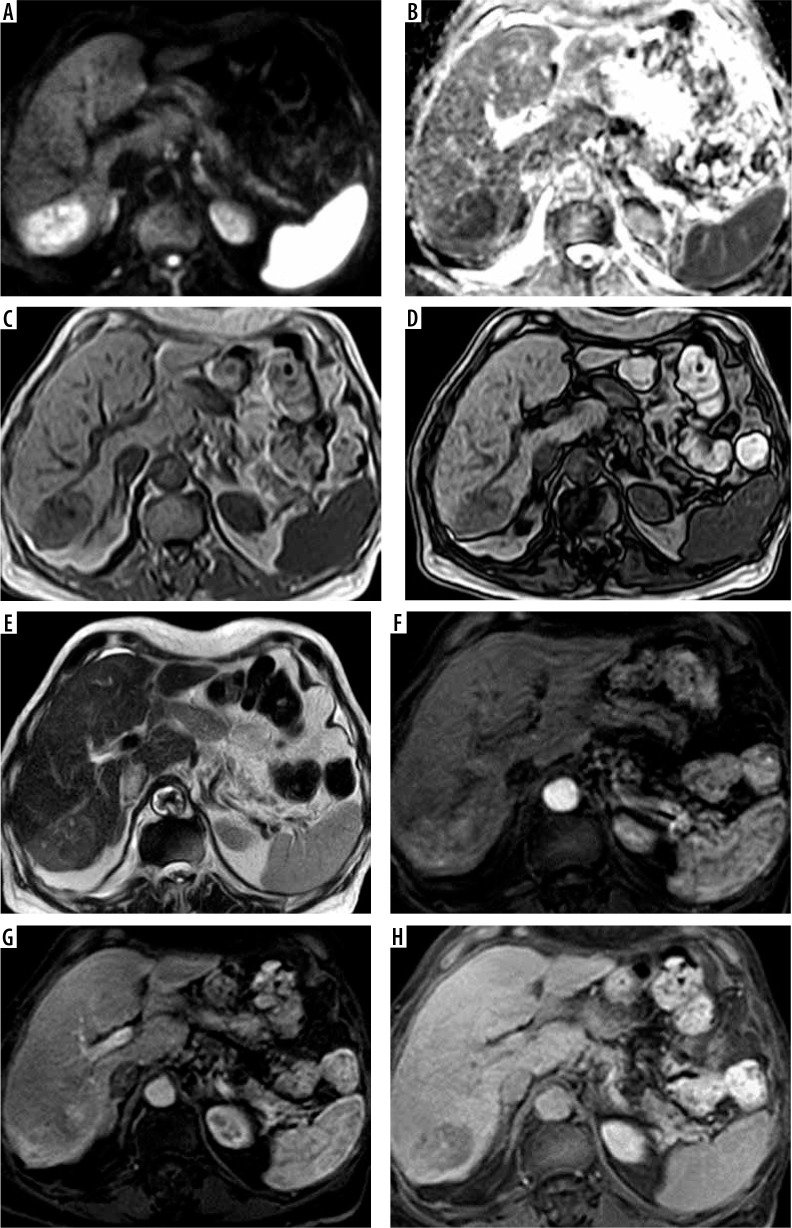
Figure 1.
A-70-year-old male patient with hepatitis C virus (HCV) infection, high a-fetoprotein, and liver cirrhosis. Full standard protocol (A, B) axial DWI and ADC showed restricted diffusion in subsegment VII lesion (C, D) axial T1 WI images in phase and out of phase: the lesion elicited a low signal. E) T2WI the lesion elicited a high signal. Dynamic contrast series (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement of the lesion with portovenous and delayed washout associated with delayed capsular enhancement. The lesion was categorized as LI-RADS-5. Abbreviated 1 protocol: E) T2WI the lesion elicited a high signal. Dynamic contrast series (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement of the lesion with portovenous and delayed washout associated with delayed capsular enhancement. The lesion was categorized as LI-RADS-5. Abbreviated 2 protocol: A, B) Axial DWI and ADC showed restricted diffusion in subsegment VII lesion. Dynamic contrast series (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement of the lesion with portovenous and delayed washout associated with delayed capsular enhancement. The lesion was categorized as LI-RADS-5. Abbreviated 3 protocol: C, D) Axial T1 WI images in phase and out of phase: the lesion elicited a low signal. Dynamic contrast series (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement of the lesion with portovenous and delayed washout associated with delayed capsular enhancement. The lesion was categorized as LI-RADS-5
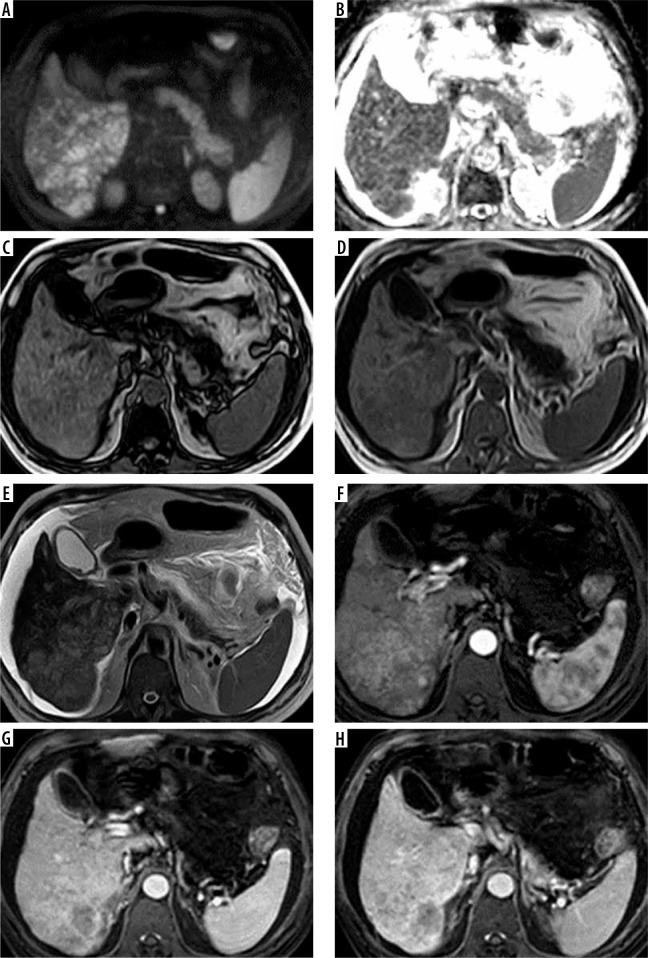
Figure 2.
A 72-year-old male patient with chronic hepatitis C virus (HCV) infection, liver cirrhosis, and high a-fetoprotein. Full standard protocol: A, B) axial DWI and ADC showed right lobe infiltrative hepatic focal lesion with true diffusion restriction. C, D) Axial T1 WI image in and out of phase: the lesion elicited a low signal. E) Axial T2WI image: the lesion elicited a high signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F) portovenous phase (G), and delayed phase (H) revealed heterogenous arterial enhancement with delayed washout of the lesion with intravenous tumoural thrombus. The lesion was categorized as LI-RADS-TIV. Abbreviated 1: E) Axial T2WI image: the lesion elicited a high signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H) revealed heterogenous arterial enhancement with delayed washout of the lesion with intravenous tumoral thrombus. The lesion was categorized as LI-RADS-TIV. Abbreviated 2: A, B) Axial DWI and ADC showed right lobe infiltrative hepatic focal lesion with true diffusion restriction. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H) revealed heterogenous arterial enhancement with delayed washout of the lesion with intravenous tumoral thrombus. The lesion was categorized as LI-RADS-TIV. Abbreviated 3: (C, D) Axial T1 WI image in and out of phase: the lesion elicited a low signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F) portovenous phase (G), and delayed phase (H) revealed heterogenous arterial enhancement with delayed washout of the lesion with intravenous tumoural thrombus. The lesion was categorized as LI-RADS-TIV
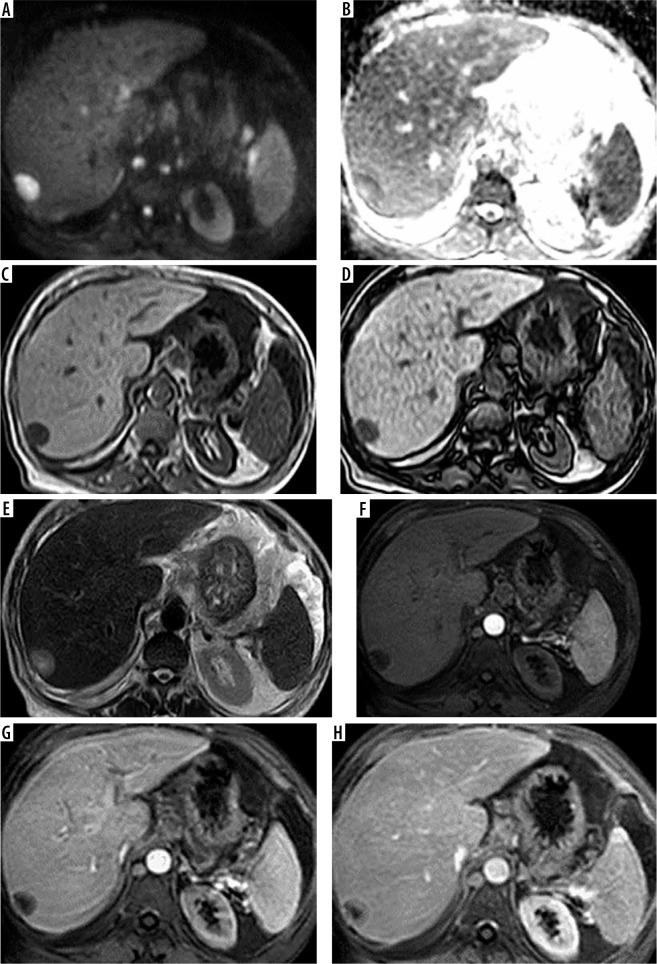
Figure 3.
A 63-year-old female patient with chronic hepatitis C virus (HCV) infection presented for screening. Full standard protocol: A, B) Axial DWI and ADC revealed a segment VII lesion with true peripheral diffusion restriction. C, D) Axial T1 WI image in and out of phase: the lesion elicited low signal. E) Axial T2WI image: the lesion elicited a high signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement “target sign” with no portovenous or delayed washout. The lesion was categorized as LI-RADS-M. Abbreviated 1: (E) Axial T2WI image: the lesion elicited a high signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement “target sign” with no portovenous or delayed washout. The lesion was categorized as LI-RADS-M. Abbreviated 2: (A, B) Revealed a segment VII lesion with true peripheral diffusion restriction. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H) revealed arterial enhancement “target sign” with no portovenous or delayed washout. The lesion was categorized as LI-RADS. Abbreviated 3: (C, D) Axial T1 WI image in and out of phase: the lesion elicited a low signal. Dynamic contrast enhancement (F, G, H): arterial phase image (F), portovenous phase (G), and delayed phase (H): revealed arterial enhancement “target sign” with no portovenous or delayed washout. The lesion was categorized as LI-RADS-M
Table 1.
The standard full protocol LI-RADS classification
| Frequency | Valid percentage | ||
|---|---|---|---|
| Valid | LR 1 | 47 | 34.06 |
| LR 2 | 9 | 6.52 | |
| LR 3 | 2 | 1.45 | |
| LR 4 | 1 | 0.72 | |
| LR 5 | 19 | 13.77 | |
| LR TIV | 12 | 8.70 | |
| LR M | 48 | 34.78 | |
| Total | 138 | 100.00 | |
Table 2.
Abbreviated 1 protocol LI-RADS classification
| Frequency | Valid percentage | ||
|---|---|---|---|
| Valid | LR 1 | 48 | 34.78 |
| LR 2 | 8 | 5.80 | |
| LR 3 | 3 | 2.17 | |
| LR 4 | 1 | 0.72 | |
| LR 5 | 19 | 13.77 | |
| LR TIV | 12 | 8.70 | |
| LR M | 47 | 34.06 | |
| Total | 138 | 100.00 | |
Table 3.
Abbreviated 2 protocol LI-RADS classification
| Frequency | Valid percentage | ||
|---|---|---|---|
| Valid | LR 1 | 47 | 34.06 |
| LR 2 | 6 | 4.35 | |
| LR 3 | 6 | 4.35 | |
| LR 4 | 1 | 0.72 | |
| LR 5 | 19 | 13.77 | |
| LR TIV | 12 | 8.70 | |
| LR M | 47 | 34.06 | |
| Total | 138 | 100.00 | |
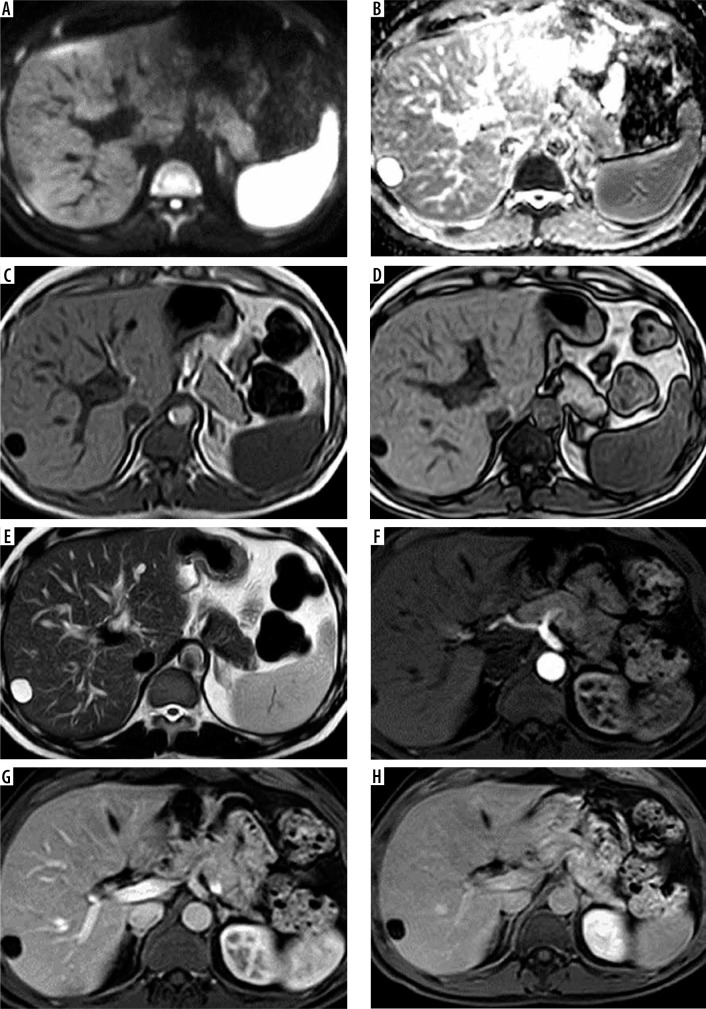
Figure 4.
A 40-year-old male patient with chronic hepatitis C virus (HCV) infection and elevated liver enzymes came for screening. Full standard protocol: A, B) DWI and ADC showed segment VII lesion with no diffusion restriction. C, D) Axial T1WI in phase and out of phase showed a lesion in hepatic subsegment VII eliciting a low signal. E) Axial T2WI: the lesion elicited a high signal. Dynamic contrast study (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) showed no enhancement of the lesion in any of them. The lesion was categorized as LI-RADS-1. Abbreviated 1: (E) Axial T2WI: the lesion elicited a high signal. Dynamic contrast study (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H): showed no enhancement of the lesion in all of them. The lesion was categorized as LI-RADS-1. Abbreviated 2: (A, B) DWI and ADC: showed segment VII lesion with no diffusion restriction. Dynamic contrast study (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H): showed no enhancement of the lesion in any of them. The lesion was categorized as LI-RADS-1. Abbreviated 3: (C, D) Axial T1WI in phase and out of phase: showed a lesion in hepatic subsegment VII eliciting a low signal. Dynamic contrast study (F, G, H): arterial phase (F), portovenous phase (G), and delayed phase (H) showed no enhancement of the lesion in any of them. The lesion was categorized as LI-RADS-3
Table 4.
Abbreviated 3 protocol LI-RADS classification
| Frequency | Valid percentage | ||
|---|---|---|---|
| Valid | LR 1 | 35 | 25.36 |
| LR 2 | 8 | 5.80 | |
| LR 3 | 17 | 12.32 | |
| LR 5 | 19 | 13.77 | |
| LR TIV | 12 | 8.70 | |
| LR M | 47 | 34.06 | |
| Total | 138 | 100.00 | |
A considerable overall k agreement between the abbreviated 1, 2, and 3 protocols on LI-RADS classification was noted with k = 0.865 (95% CI: 0.814-0.917; p < 0.001) (Table 5). Subsequently, there was almost perfect agreement between all abbreviated protocols and full standard protocol on LI-RADS classification, with k = 0.890 (95% CI: 0.853-0.927) (p < 0.001) (Table 6).
Table 5.
Abbreviated 1, 2, and 3 protocols LI-RADS classification overall kappa (κ) agreement
| κ | Asymptotic standard error | Z | p-value | Lower 95% asymptotic CI bound | Upper 95% asymptotic CI bound | |
|---|---|---|---|---|---|---|
| Overall | 0.865 | 0.026 | 32.984 | 0.000 | 0.814 | 0.917 |
Table 6.
Abbreviated 1, 2, 3, and full standard reference protocols LI-RADS classification overall kappa (κ) agreement
| κ | Asymptotic standard error | Z | p-value | Lower 95% asymptotic CI bound | Upper 95% asymptotic CI bound | |
|---|---|---|---|---|---|---|
| Overall | 0.890 | 0.019 | 47.490 | 0.000 | 0.853 | 0.927 |
In a comparison between the standard reference full protocol and each abbreviated protocol regarding the k agreement on LI-RADS classification, there was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol with k = 0.980 (95% CI: 0.949-1; p < 0.001) (Table 7). While the abbreviated 2 protocol showed good but lesser agreement with the full protocol, with k = 0.961 (95% CI: 0.92-0.99; p < 0.001) (Table 8). On the other hand, the abbreviated 3 protocol show-ed a good, but the least k, agreement with the full protocol, with k = 0.81 (95% CI: 0.736-0.882; p < 0.001) (Table 9).
Table 7.
Agreement between standard reference and abbreviated 1 protocol
| Value | Bias | Std. error | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Measure of agreement | κ | 0.980 | –0.001 | 0.014 | 0.949 | 1.000 | 0.000p < 0.001 |
Table 8.
Kappa agreement between standard reference and abbreviated 2 protocol
| Value | Bias | Std. error | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Measure of agreement | κ | 0.961 | 0.000 | 0.019 | 0.920 | 0.990 | 0.000p < 0.001 |
Table 9.
Kappa agreement between standard reference and abbreviated 3 protocol
| Value | Bias | Std. error | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Measure of Agreement | κ | 0.810 | 0.000 | 0.036 | 0.736 | 0.882 | 0.000p < 0.001 |
The abbreviated 1 and 2 protocols showed high diagnostic performance on LI-RADS classification of lesions, with 100% sensitivity, specificity, PPV, NPV, and accuracy using the full protocol as a standard reference, while the abbreviated 3 protocol showed a lesser but comparable sensitivity of 96.9%, NPV 99.4%, and accuracy 99.4%. However, it revealed the same PPV and specificity (Table 10).
Table 10.
Diagnostic performance of abbreviated protocols on LI-RADS classification of lesions
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Abb 1 | 100% | 100% | 100% | 100% | 100% |
| Abb 2 | 100% | 100% | 100% | 100% | 100% |
| Abb 3 | 96.9% | 100% | 100% | 99.3% | 99.4% |
Discussion
Abbreviated protocols can shorten the acquisition time and shorten the time needed for interpretation and reporting by the radiologist. Furthermore, evaluation of a lesser imaging series helps the radiologist to maintain focus and productivity [5].
Although few studies have evaluated the dynamic abbreviated MRI for screening, a growing body of literature recommends it as a suitable tool for HCC diagnosis, with a high per-patient sensitivity of 85-92% for HCC and specificity of 89-100% [5].
In our study, we compared the diagnostic performance of different abbreviated MRI protocols in screening for HCC, but we could not dispense with dynamic contrast sequences due to the high risk of HCC in our country as one of the countries with a heavy HCV burden.
In our study the abbreviated 1 and 2 protocols showed high diagnostic performance on LI-RADS classification of lesions with 100% sensitivity, specificity, PPV, NPV, and accuracy using the full protocol as a standard reference, while the abbreviated 3 protocol showed a lesser but comparable sensitivity of 96.9%, NPV 99.4, and accuracy 99.4%. However, it revealed the same PPV and specificity. Bruising et al. in 2020 [6] reported comparable results on several studies that retrospectively assessed the diagnostic performance of non-contrast abbreviated MRI protocols. While these studies found favourable sensitivities ranging from 84% to 92% on a per-patient basis, they used liver pathology as the reference standard.
Hecht et al. in 2006 [7] used only contrast-enhanced T1-weighted imaging in a retrospective study as an abbreviated technique for detection of HCC and reported a lesser sensitivity of 68.4% and lesser specificity of 65.7% than the 3 abbreviated protocols in our study. Additionally, McNamara in 2018 [8] used DWI alone for an abbreviated protocol and demonstrated a lesser sensitivity of 78% and specificity of 88%. However, he used pathology as the gold standard for diagnosis. Another prospective study was done by Sutherland in 2016 [9], which compared abbreviated techniques using only DWI to the ultrasound as a standard reference and revealed a lesser but still comparable sensitivity and specificity of 83% and 98%, respectively.
Our study was in concordance with results reported by Ahmed et al. in 2020 [10], who used only T2WI/T2-SPIR and reported a comparable sensitivity of 86.67% and specificity of 100%. They also reported that adding the DWI/ADC into the abbreviated protocol increased the sensitivity to 100% and retained specificity at 100%. A retrospective study was done by Khatri et al. in 2020 [11], which assessed the performance of a dynamic abbreviated protocol for HCC detection using the full protocol as a standard reference and revealed comparable results to our study with sensitivity and specificity of 92.1% and 88.6%, respectively. Besa et al. in 2017 [12] demonstrated a comparable sensitivity (80.6) using DWI with a T1W delayed-phase postcontrast protocol. Also, a study was done by Marks et al., [13] combining T1W delayed post-contrast and T2W imaging, in which the sensitivity was observed to be 82.6%. A meta-analysis done by Gupta et al. in 2021 [3] evaluated the accuracy of all abbreviated MRIs for HCC screening and reported comparable sensitivity and specificity of 86% and 94%, respectively.
In the first protocol we used T2WI with dynamic contrast MRI and compare it with the full standard protocol, and there was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol, with k = 0.980 (95% CI: 0.949-1; p < 0.001). In the second protocol we used DWI with dynamic contrast MRI and compared it with the full standard protocol, it showed a good but lesser agreement with the full protocol, with k = 0.961 (95% CI: 0.92-0.99; p < 0.001). On the other hand, the abbreviated 3 protocol, in which we used T1 in and out of phase associated with dynamic contrast MRI comparing it with the full standard protocol, showed a good, but the least k, agreement with the full protocol, with k = 0.81 (95% CI: 0.736-0.882; p < 0.001). Our study was in concordance with the results obtained by McNamara. In 2018 [8] using DWI alone for an abbreviated protocol, which reported that there was no significant difference between the DWI and complete full study, with a k coefficient of 0.6716. Another retrospective study was done by Khatri et al. in 2020 [11], which assessed the performance of a dynamic abbreviated protocol for HCC detection and revealed comparable results to our study with good agreement probability between abbreviated MRI and full protocol.
There was an almost perfect agreement between all abbreviated protocols and full standard protocol on LI-RADS classification, with K = 0.890 (95% CI: 0.853-0.927; p < 0.001). In a comparison between the standard reference full protocol and each abbreviated protocol regarding the k agreement on LI-RADS classification, there was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol, with a total duration of this abbreviated protocol of 8 min 48 sec compared to at least 18 min for the full standard protocol. Hence, we can conclude by recommending use of this protocol as a standard protocol for screening high-risk patients.
There were several limitations in our study because it was conducted on a 1.5 T MRI machine. Moreover, we excluded patients with already diagnosed viable HCC and/or HCC treated with chemoembolization. Additionally, we did not compare our results to histopathology, but we used the full standard protocol as a reference.
Conclusion
Abbreviated contrast-enhanced MRI protocols can be used as a screening tool for the detection of HCC with high sensitivity, specificity, PPV, NPV, and accuracy close to that of the full protocol. There was a considerable highest agreement between the abbreviated 1 protocol and the full standard protocol, with a total duration of 8 min 48 sec compared to at least 18 min for the full standard protocol. Hence, this protocol can be used as a cost-effective protocol for screening high-risk patients, with short scanning time and with high diagnostic performance.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Chan MV, McDonald SJ, Ong YY, et al. HCC screening: assessment of an abbreviated non-contrast MRI protocol. Eur Radiol Exp 2019; 3: 49. doi: 10.1186/s41747-019-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canellas R, Rosenkrantz AB, Taouli B, et al. Abbreviated MRI protocols for the abdomen. Radiographics 2019; 39: 744-758. doi: 10.1148/rg.2019180123. [DOI] [PubMed] [Google Scholar]
- 3.Gupta P, Soundararajan R, Patel A, et al. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J Hepatol 2021; 75: 108-119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Huo EJ, Weinstein S, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol 2018; 43: 1627-1633. doi: 10.1007/s00261-017-1339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An J, Peña MA, Cunha GM, et al. Abbreviated MRI for hepatocellular carcinoma screening and surveillance. Radiographics 2020; 40: 1916-1931. doi: 10.1148/rg.2020200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunsing RL, Fowler KJ, Yokoo T, et al. Alternative approach of hepatocellular carcinoma surveillance: abbreviated MRI. Hepatoma Res 2020; 6: 59. doi: 10.20517/2394-5079.2020.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht EM, Holland AE, Israel GM, et al. Hepatocellular carcinoma in the cirrhotic liver: gadolinium-enhanced 3D T1-weighted MR imaging as a stand-alone sequence for diagnosis. Radiology 2006; 239: 438-447. doi: 10.1148/radiol.2392050551. [DOI] [PubMed] [Google Scholar]
- 8.McNamara MM, Thomas J v., Alexander LF, et al. Diffusion-weighted MRI as a screening tool for hepatocellular carcinoma in cirrhotic livers: correlation with explant data–a pilot study. Abdom Radiol 2018; 43: 2686-2692. doi: 10.1007/s00261-018-1535-y. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland T, Watts J, Ryan M, et al. Diffusion-weighted MRI for hepatocellular carcinoma screening in chronic liver disease: direct comparison with an ultrasound screening. J Med Imaging Radiat Oncol 2017; 61: 34-39. doi: 10.1111/1754-9485.12513. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed NNA, el Gaafary SM, Elia RZ, Abdulhafiz EM. Role of abbreviated MRI protocol for screening of HCC in HCV-related cirrhotic patients prior to direct-acting antiviral treatment. Egyptian Journal of Radiology and Nuclear Medicine 2020; 51. doi: 10.1186/s43055-020-00199-x. [DOI] [Google Scholar]
- 11.Khatri G, Pedrosa I, Ananthakrishnan L, et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J Magn Reson Imaging 2020; 51: 415-425. doi: 10.1002/jmri.26835. [DOI] [PubMed] [Google Scholar]
- 12.Besa C, Lewis S, Pandharipande P, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol 2017; 42: 179-190. doi: 10.1007/s00261-016-0841-5. [DOI] [PubMed] [Google Scholar]
- 13.Marks RM, Ryan A, Heba ER, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. Am J Roentgenol 2015; 204: 527-535. doi: 10.2214/AJR.14.12986. [DOI] [PubMed] [Google Scholar]