Fig. 3. MTCH2 inserts diverse mitochondrial TAs into the outer membrane.
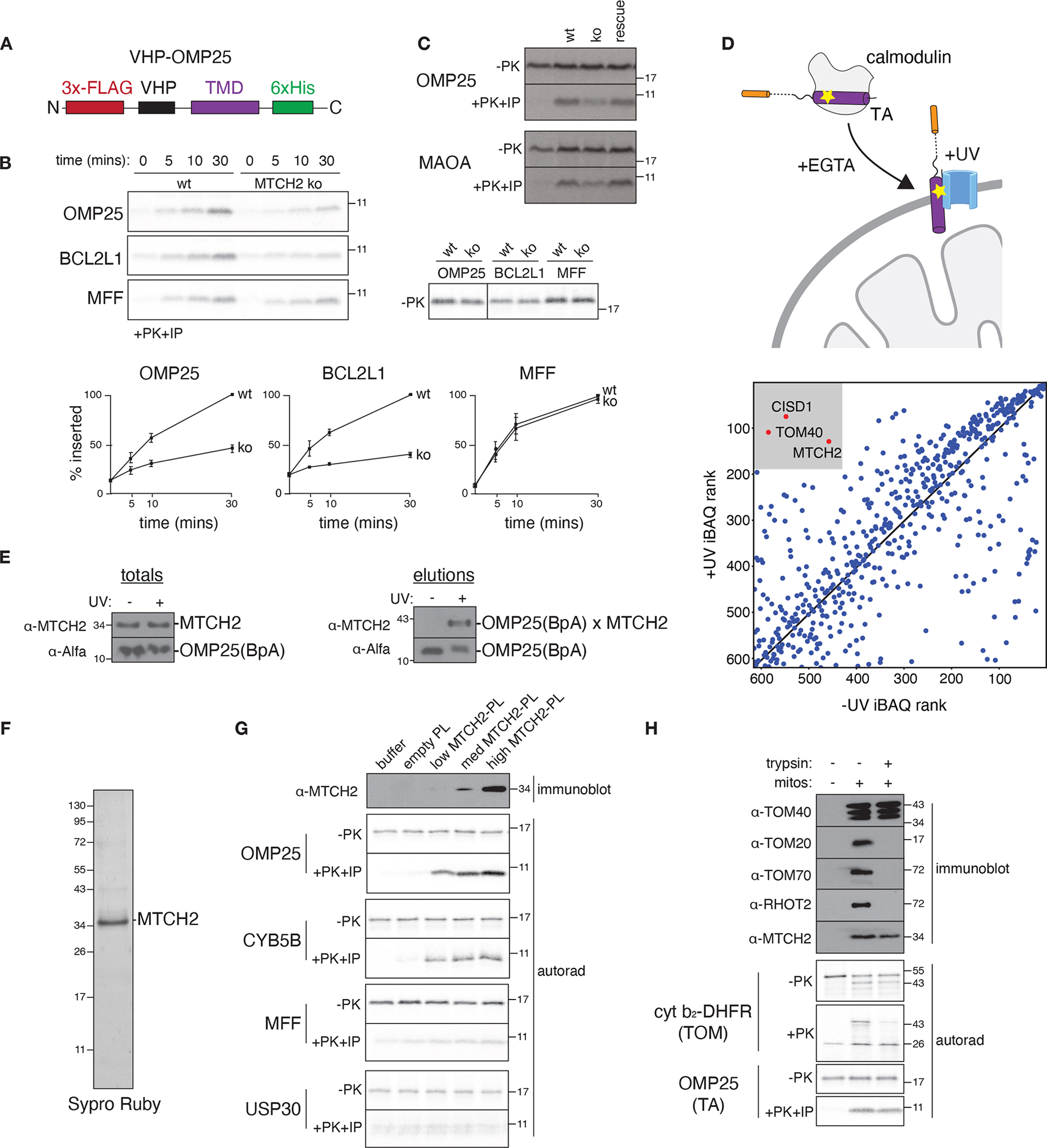
(A) Schematic of the fusion between an inert N-terminal globular protein (VHP) and the TMDs of a panel of mitochondrial TAs (see also fig. S9) generated to probe TMD dependent insertion by MTCH2. (B) The indicated 35S-methionine labelled TA proteins were analyzed for in vitro insertion over time into mitochondria isolated from wild type (wt) or MTCH2 knockout (ko) K562 cells. Displayed are the samples prior to addition of protease (−PK; top right) and the protease protected fragment that has been affinity purified via a 6xHIS tag on the C-terminus of each substrate (+PK+IP; top left), ensuring insertion in the correct topology. (Bottom) Quantification of three biological replicates are plotted with error bars indicating one standard deviation at each time point. (C) As in (B) comparing insertion of the indicated TA proteins into wild type, MTCH2 ko, and MTCH2 ko + MTCH2 rescue mitochondria. (D) (Top) Schematic showing the photocrosslinking strategy. OMP25 containing the photoactivatable amino acid BpA within its TMD was expressed and purified from E. coli as a complex with calmodulin. OMP25BpA was released from calmodulin by addition of EGTA in the presence of mitochondria purified from K562 cells using a percoll gradient (fig. S1B). Crosslinking was activated by UV-irradiation, and the resulting crosslinked species were affinity purified via the Alfa-tag on the N-terminus of OMP25BpA for identification by mass spectrometry. (Bottom) All proteins identified by mass spectrometry were ranked by iBAQ abundance, and those specifically enriched in the UV compared to the -UV control are highlighted. Though TOM40 and CISD1 were identified, they were not significant hits in our screen (fig. S12), while TOM40 was not required for biogenesis both in vitro (Fig. 1A) and in cells (fig. S12B) (E) As in (D) with the resulting elution analyzed by immunoblotting to assess levels of crosslinked OMP25 BpA-MTCH2. (F) MTCH2 was expressed and purified from human cells and analyzed by SDS-PAGE and Sypro-Ruby staining. (G) Following reconstitution (see fig. S13 for optimization of conditions), the recovered proteoliposomes were analyzed by immunoblotting for incorporation of MTCH2. Using a protease protection assay, the indicated MTCH2 dependent (OMP25, CYB5B) and independent (MFF, USP30) 35S methionine labelled substrates synthesized in rabbit reticulocyte lysate were tested for insertion into liposomes reconstituted with increasing amounts of purified MTCH2 compared to an empty control. The resulting protease protected fragments were immunoprecipitated, imaged by autoradiography (autorad). (H) Mitochondria from wt K562 cells were treated with trypsin and their ability to insert TOM (Su9-DHFR) or TA substrates (OMP25) was assayed by protease protection as in (A). The indicated outer membrane proteins were confirmed to be degraded in a trypsin-dependent manner by immunoblot, while MTCH2 remained largely intact.
