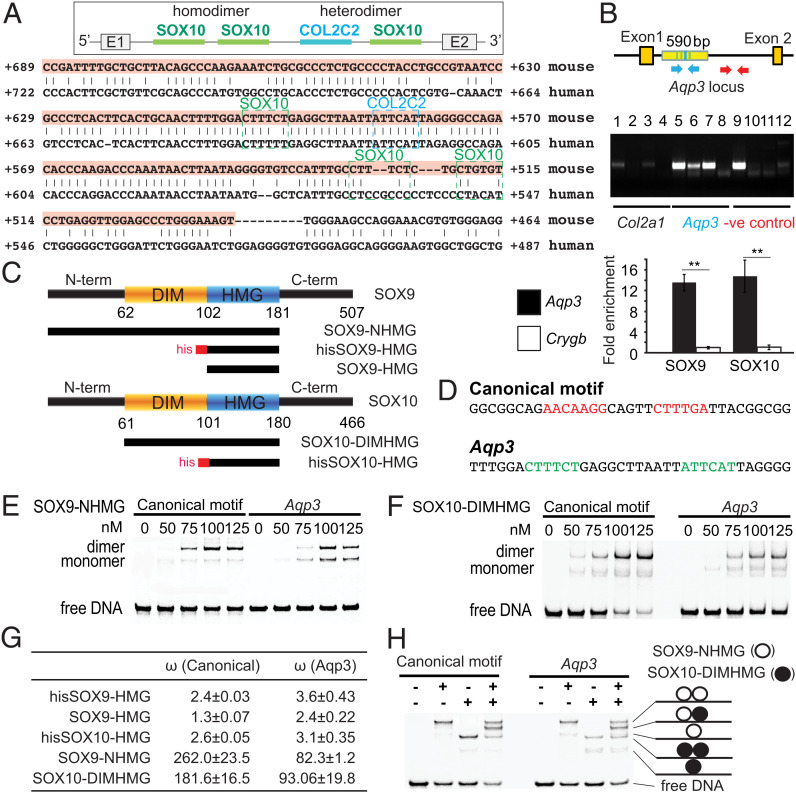
Fig. 5.
Aqp3 is potentially regulated by SOXE dimers with strong positive cooperativity. (A) Conserved SOX9 and SOX10 binding sites predicted by bsConserve and Jasper over a 205-bp conserved noncoding region by Vista in intron 1 of Aqp3. A COL2C2 (ATTCAT) SOX9 binding site (69) is detected in intron 1. COL2C2 and SOX10 binding sites are boxed in blue and green, respectively. (B) ChIP assays for SOX9 and SOX10 on the WT inner ear at E14.5 (n = 2). Col2a1 (positive control) is the known SOX9 binding region. Arrows in blue indicate primer sites flanking the Aqp3 intron 1 region with predicted SOX9 and SOX10 binding sites. Arrows in red indicate primer sites flanking 1 kb downstream of the predicted SOX9 or SOX10 binding site. Real-time PCR-amplified DNA fragments were immunoprecipitated by SOX9 or SOX10 antibody, the Aqp3 intron1 region (blue arrows), or the Crygb (gamma-b-crystallin) promoter (negative control; n = 3). Lanes 1, 5, and 9: genomic DNA (gDNA); lanes 2, 6, and 10: α-SOX10 antibody (Ab); lanes 3, 7, and 11: α-SOX9 Ab; lanes 4, 8, and 12: control serum. (C) Domain composition of SOX9 and SOX10 proteins and (D) DNA sequences of oligos used in EMSAs. (E) The SOX9-NHMG and (F) SOX10-DIMHMG constructs containing the DIM domain dimerized with strong positive cooperativity (n = 4). (G) The homodimer cooperativity factors were estimated as described (98, 99). (H) SOX9 and SOX10 proteins heterodimerized effectively on the Aqp3 sequences (n = 4). -, not added. +, added. Data are presented as means ± SEM. **P < 0.01.