Abstract
Stress affects many brain regions, including the ventral tegmental area (VTA), which is critically involved in reward processing. Excessive stress can reduce reward-seeking behaviors but also exacerbate substance use disorders, two seemingly contradictory outcomes. Recent research has revealed that the VTA is a heterogenous structure with diverse populations of efferents and afferents serving different functions. Stress has correspondingly diverse effects on VTA neuron activity, tending to decrease lateral VTA dopamine (DA) neuron activity, while increasing medial VTA DA and GABA neuron activity. Here we review the differential effects of stress on the activity of these distinct VTA neuron populations and how they contribute to decreases in reward-seeking behavior or increases in drug self-administration.
Keywords: GABA, Dopamine, Anhedonia, Substance use
Introduction
The ventral tegmental area (VTA) is a midbrain structure which plays a significant role in regulating motivated behavior. Dopamine (DA) and GABA neurons in the VTA act together to encode reward prediction error and reward anticipation [1], and drugs of abuse can act on these neurons to promote substance use disorders [2,3]. Thus, altered VTA activity as a consequence of stress, can impact reward-seeking behavior. While stress can elicit adaptive responses (marshalling resources to cope with challenges such as illness), excessive stress can prove maladaptive [4,5]. Acute stressors can decrease reward-seeking behavior, and exposure to chronic stress can produce lasting deficits in reward-seeking similar to anhedonia, a symptom defined as “markedly diminished interest or pleasure in all, or almost all activities most of the day, nearly every day” commonly seen in major depressive disorder (MDD) [6–8]. By contrast, stress can exacerbate substance use disorders [9]. In this review, we will discuss the effects of stress on the activity of different VTA neurons and the possible consequences for motivated behavior.
Stress impairs reward anticipation
As the VTA plays an important role in reward processing, stress modulation of VTA activity has many consequences for reward-seeking. Restraint stress, where an animal is stressed by confinement/immobilization, impairs motivation as measured by reduced breakpoint in a progressive ratio task [10]. In a progressive ratio task, an animal must perform a behavior (e.g., a lever press) a certain number of times before earning a reward. With each reward earned, the number of behaviors required gets progressively larger until the number of actions required exceeds the motivation of the animal to perform the task. This final reward schedule is called the breakpoint. Consistent with this stress-induced reduction of motivation, we recently found that restraint stress impairs cue-evoked reward-seeking behavior in a Pavlovian reward task [11].
Stress differentially affects reward learning
While evidence suggests that stress impairs reward-seeking behavior, there is also evidence that stress can facilitate learning new reward tasks. A single exposure to restraint stress increases learning of a cued-reward task via increased dopamine release in the ventrolateral striatum [12]. Notably, the stress that produces this effect is delayed as a 20-min pause is introduced between stress and the onset of training and training continues for 10 days without stress. By contrast, more immediate short-term stress can impair reward learning. In human subjects, acute stress in the form of threat of electric shock, negative performance feedback, or final exam stress, prevents subjects from learning which stimulus is more frequently rewarded [13–15]. The influential Yerkes-Dodson theory proposes that the effects of stress follows an inverted U-shaped curve with intermediate stress producing better outcomes than either no or excessive stress [5]. Thus, it seems likely that immediate versus delayed stressors impose different levels of stress and thereby fall at different points on the inverted-U shaped curve.
Stress promotes drug self-administration
In contrast to the stress-induced reductions in reward anticipation and learning, stress can increase drug misuse. Mice subjected to a single episode of social defeat stress, during which larger, more aggressive mice physically attack them, develop an increased preference for a chamber paired with cocaine (conditioned place preference), indicating that they find cocaine more rewarding [16]. In addition to promoting conditioned place preference for cocaine, there is converging evidence that stress increases drug self-administration. Stress increases ethanol self-administration in rats [17] and chronic social defeat stress increases cocaine self-administration in both mice and rats [18–20]. That stress paradoxically reduces reward-seeking while enhancing self-administration of drugs of abuse highlights the importance of understanding the impact of stress on the underlying neural circuitry.
CRF and glucocorticoids in the VTA
Stressful experiences activate the hypothalamic-pituitary-adrenal (HPA) axis by inducing release of corticotropin releasing factor (CRF) from the hypothalamus, which in turn stimulates the pituitary gland to release adrenocorticotropic hormone, which stimulates the adrenal glands to release the glucocorticoid cortisol in humans (or corticosterone in rodents). In fact, exogenous CRF administration can largely recapitulate the physiological and behavioral impacts of stress [21]. By binding to mineralocorticoid and glucocorticoid receptors, cortisol and corticosterone act broadly in the body to respond to stressors, including directly in the VTA [22]. For example, glucocorticoid receptors in the VTA are necessary for the fasting-induced palatability of high-fat food and corticosterone administration can potentiate glutamatergic synapses on VTA DA neurons via glucocorticoid receptors [23]. Mineralocorticoid receptors, by contrast, modulate fear conditioning [23,24] and regulate dopamine release in the nucleus accumbens (NAc) and the basolateral amygdala [25–27]. However, most research has focused on the effects of CRF, rather than corticosterone, in the VTA, as CRF-expressing neurons in the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus have all been shown to project to the VTA [28–30], and a subset of VTA DA neurons express CRF [31].
Infusing CRF into the VTA decreases the progressive ratio breakpoint, whereas intra-VTA infusion of a nonselective CRF antagonist prevents restraint stress from reducing the breakpoint [10]. CRF can bind to two different receptors in the VTA, CRFR1 and CRFR2. CRFR1 activation in the VTA is associated with increased ex vivo tonic activity of putative VTA DA neurons [32]. Higher levels of CRFR1 expression are associated with lower anxiety and greater motivation in rats [33], and acute stress can increase CRFR1 expression in the VTA [34]. In addition, CRFR1 activation increases the ex vivo release probability of VTA GABA neurons and can increase evoked inhibitory postsynaptic current (IPSC) amplitude or spontaneous IPSC frequency on VTA DA neurons [35]. CRFR2 activation, by contrast, can potentiate NMDA receptors on putative VTA DA neurons [36]. Similarly, CRFR2 activity and phenylephrine (an agonist of α1-adrenoceptors) cooperatively increase long-term potentiation (LTP) of glutamatergic input on putative VTA dopamine neurons [16].
Most of the above evidence comes from ex vivo experiments, so it remains unclear how these mechanisms interact during stress-induced impairments to reward-seeking and escalation and reinstatement of drug use. However, one pattern that emerges is that stress decreases baseline VTA DA neuron activity and increases baseline VTA GABA neuron activity. At the same time, stress has the opposite effect on the plasticity of inputs to VTA DA neuron plasticity, for example, enhancing glutamatergic synapses. This opposing impact of stress on baseline VTA neural activity and plasticity may help explain the divergent effects of stress on reward-seeking and drug self-administration. For further discussion of the implications of stress-induced plasticity on drug-seeking, see below.
Stress effects on endogenous opioids and melanocortin in the VTA
Stress can impact reward behaviors by targeting the endogenous opioid system in the VTA. Stress can produce release of the endogenous opioid dynorphin, which activates the κ opioid receptor (κOR) [37]. The lateral hypothalamus is a source of dynorphin in the VTA [38]. Exogenous application of dynorphin decreases the ex vivo firing rate and frequency of spontaneous excitatory postsynaptic current (EPSC) on VTA DA neurons [39–41], thus the net effect of stress activation of is VTA κORs is to decrease DA release.
Stress can also alter μ-opioid receptor (μOR) signaling in the VTA. Stress induces analgesia via the release of endorphins and consequent activation of μORs [42]. The impact of stress on reward behavior is linked to altered melanocortin signaling derived from the same neurons as endogenous μOR ligands. Chronic restraint stress increases the ex vivo firing rate of proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH) [43]. In addition to releasing GABA and/or glutamate, ARH POMC neurons can also release neuropeptides like β-endorphin, which preferentially activates μORs, or α-melanocyte-stimulating hormone (α-MSH), which activates the melancortin-3 and melanocortin-4 receptors (MC3R and MC4R) [44]. Optogenetic activation of POMC ARH neurons directly inhibits VTA DA neurons by activating μORs with β-endorphin. POMC ARC stimulation can also indirectly inhibit VTA DA neurons via VTA GABA excitation, an effect blocked by a melanocortin receptor antagonist [43]. Inhibiting POMC ARH terminals in the VTA during chronic restraint stress (one hour of restraint stress per day for 14 days) rescues sucrose preference, forced swim, and tail suspension behaviors [43]. Thus, stress-activation of POMC ARH neurons may contribute to the effects of stress on behavior by both directly and indirectly inhibiting VTA DA neurons.
Stress inhibits lateral VTA DA neurons and excites medial VTA DA neurons
Different studies have found that stress or aversive stimuli can excite [45–48], inhibit [11,43,49–52], or bidirectionally modulate VTA DA neurons [53–57]. This discrepancy in results likely reflects different populations of VTA DA neurons [58], different modalities of stress [59], or whether acute or chronic stress is administered. Further studies are needed to explain these discrepancies, although a general pattern has emerged in which stress appears to inhibit lateral VTA DA neurons and excite medial VTA DA neurons. There is an anatomical division of the projection targets of VTA DA neurons, such that medial VTA DA neurons tend to project to the medial NAc and lateral VTA DA neurons tend to project to the lateral NAc [60]. A recent study looking at in vivo calcium activity in DA terminals in the NAc while mice underwent a cued-footshock found that aversive stimuli excite medial shell-projecting VTA DA terminals, but inhibit lateral shell-projecting VTA DA terminals [56]. Interestingly, these lateral shell-projecting DA neurons were excited above baseline levels after footshock termination, consistent with a past in vivo microdialysis study showing post-stress elevation in dopamine levels [61]. Collectively, it appears that stress can excite and inhibit different populations of VTA DA neurons depending on their projection target.
Several studies support the hypothesis that stress inhibits lateral VTA DA neurons. We recently reported that restraint stress inhibits DA neurons in the lateral VTA during the stressor [11]. Chronic mild stress, a form of chronic stress where researchers expose rodents to a variety of stressors (e.g., food deprivation, continuous lighting, cage tilting) over the course of weeks, decreases the in vivo and ex vivo firing rate of lateral VTA DA neurons via downregulation of specific voltage-gated ion channels knockdown or over-expression of these channels in the VTA can bidirectionally modulate depressive-like behaviors [62]. Consistent with this finding, in vivo optogenetic stimulation or inhibition of lateral VTA DA neurons respectively increases or decreases reward behavior [63]. Ex vivo studies also show that stress enhances ethanol-induced spontaneous IPSC frequency in lateral VTA DA neurons [17]. Various studies have also demonstrated effects of stress on brain regions that target lateral VTA DA neurons. Acute unpredictable foot shock stress, a stressor in which mice are exposed to footshocks at pseudo-random intervals over a 20 min period, increases ex vivo glutamate release from lateral habenula (LHb) neurons onto the rostromedial tegmental nucleus (RMTg), where LHb neurons preferentially synapse upon GABA neurons that in turn inhibit lateral VTA DA neurons [64,65].
Of note, not all stress-modulated projections to lateral VTA DA neurons are consistent with decreased activation of lateral VTA DA neurons. For example, the laterodorsal tegmentum (LDTg) projects to lateral VTA DA neurons and is necessary for VTA DA bursting [65,66]. However, acute and chronic social defeat stress increase the excitability of VTA-projecting laterodorsal tegmental (LDTg) neurons, which in turn increases ex vivo VTA DA neuron excitability [67]. There is also evidence that chronic restraint stress can activate excitatory projections from the deep cerebellar nuclei (DCN) to the lateral VTA DA neurons [68]. Further in vivo research is needed to integrate these effects of stress on LDTg and DCN inputs to VTA DA neurons with other influences on VTA DA neuron activity. For instance, stress-induced inhibition of lateral VTA DA neuron activity may be due to voltage gated channel downregulation, feedforward inhibition through LHb activation of the RMTg, or VTA GABA activation (see below).
Aversive stimuli excite medial NAc-projecting VTA DA terminals and increase DA release in the medial NAc [56,69]. This effect appears to be mediated by glutamatergic inputs from the lateral hypothalamus (LH), which are activated by aversive cues and which preferentially target medial NAc-projecting VTA DA neurons [56]. In addition, chronic mild stress increases the in vivo firing rate of VTA-projecting LHb neurons in stress-susceptible mice [70]. There is evidence these LHb neurons project to DA neurons in the medial VTA which in turn project to the medial PFC, but not the medial NAc [65]. In female rats, early life stress in the form of maternal separation for 3 h during postnatal days 2–14 increases the early adulthood ex vivo excitability of medial VTA DA neurons, as well as the amplitude and frequency of spontaneous EPSCs in both medial and lateral VTA DA neurons [71].
Stress can also influence inhibitory transmission onto VTA DA neurons. While LTP is often discussed in the context of glutamatergic synapses, it can also occur at GABAergic synapses (LTPGABA), whereby activation of glutamatergic NMDA receptors results in increased IPSC magnitude, likely via increased GABAA receptor expression [72,73]. Interestingly, stress blocks LTPGABA on putative VTA DA neurons ex vivo [74]. Over time, this blockade of LTPGABA might be expected to reduce IPSC amplitudes, although a single episode of stress does not appear sufficient to affect IPSC amplitude on VTA DA neurons [17]. This effect of stress may predominantly impact lateral shell-projecting VTA DA neurons, as ex vivo recordings suggest that lateral shell-projecting VTA DA neurons receive more IPSCs than medial shell-projecting VTA DA neurons [60]. Thus, stress appears to increase medial VTA DA neuron firing rates by activating glutamatergic projections from the LH and LHb, enhancing LTP at glutamatergic synapses, and reducing LTP at GABAergic synapses.
As discussed above, lateral VTA DA neurons support reward seeking, while medial VTA DA neurons respond equally well to rewarding and aversive stimuli [75]. Thus, this net pattern of lateral VTA DA neuron inhibition and medial VTA DA neuron activation may represent a shift toward devaluing rewards and promoting avoidance behavior. Consistent with this framework, activating the lateral VTA DA circuit produces conditioned place preference whereas medial VTA DA circuit activation promotes conditioned place avoidance [65].
Stress activates VTA GABA neurons
While VTA DA neurons have been the central focus of stress-induced disruptions in reward seeking for decades [76], recent studies have highlighted the role of VTA GABA neurons [2]. Studies of the effect of stress on VTA GABA neurons converge on the finding that stress excites VTA GABA neurons, albeit at different timescales. The delivery of brief aversive stimuli, including air puffs, footshocks, and looming stimuli, all excite VTA GABA neurons in vivo [45,77,78]. Recently we showed that stress inhibits in vivo VTA GABA neurons during the stressor, but increases reward cue-evoked activity of VTA GABA neurons after stress [11].
Different mechanisms have been proposed for the short- and long-term effects of stress on VTA GABA activity. Looming stimuli transiently excite VTA GABA neurons in vivo via excitatory inputs from the superior colliculus [78]. A recent study also suggests that chronic stress can activate excitatory inputs from the DCN to VTA GABA neurons [68]. Newly emerging evidence shows heterogenous subpopulations of VTA GABA neurons [2] and future studies should investigate how these subpopulations differentially respond to stress and interact with the heterogenous subpopulations of VTA DA neurons.
Stress modulates VTA neural responses to drugs of abuse
Given that the net influence of stress-induced activity (decreased lateral VTA DA neuron activity, increased medial VTA DA and GABA neuron activity) supports decreased reward seeking, it seems puzzling that stress promotes drug self-administration. Perhaps the key to this conundrum lies in the plastic changes induced by both drugs of abuse and stress. All drugs of abuse directly modulate the activity of VTA projections to the NAc, and drug addiction is hypothesized to form through synaptic plasticity at VTA DA synapses [79]. Similarly, stress induces synaptic plasticity at VTA DA synapses [16,23,48,58,80–82]. Thus, stress-induced enhancements of drug self-administration may reflect the interaction of these plastic processes, a form of meta-plasticity [83] in which the pre-existing plasticity induced by drugs of abuse is enhanced by stress-induced plasticity. Consistent with this hypothesis, CRFR1 activity enhances cocaine-induced DA release in the NAc, as well as stress-induced increase of cocaine self-administration [18,19,84,85]. CRFR2 is associated with stress-induced reinstatement of cocaine self-administration after extinction as well as potentiation of NMDA receptors on putative VTA DA neurons [36,86]. There is also evidence that CRFR2 activation may act cooperatively with norepinephrine signaling in the VTA to enhance cocaine conditioned place preference by increasing glutamatergic LTP on putative VTA dopamine neurons [16]. Stress also inhibits LTPGABA at inhibitory synapses of VTA DA via increased constitutive activity of κORs, which contributes to stress-induced cocaine reinstatement [20]. This impaired LTPGABA is similar to the effect of a single exposure of morphine, cocaine, ethanol, or nicotine [73,87–89]. One particularly interesting mechanism whereby stress promotes ethanol self-administration has been proposed in which stress or corticosterone alters the reversal potential of chloride in VTA GABA neurons such that ethanol leads to increased VTA GABA firing rate and blunted VTA DA response [17]. These findings raise the possibility that stress and/or drugs of abuse can alter the fundamental function of reward circuit elements. Further research is needed to directly test how stress- and drug-induced plasticity interact and how they influence the pursuit of drugs of abuse versus food or other naturally occurring rewards within individual subjects. Similarly, a key open question is whether distinct populations of VTA neurons (with potentially different sensitivity to stress) mediate the response to different types of drugs of abuse and rewards.
Stress increases neuromodulatory input to the VTA
As stress has widespread effects on neuronal activity, understanding the effects of stress on neurons projecting to the VTA can provide additional insight into how stress alters VTA activity at a projection-specific level (Figure 1).
Figure 1. Summary of the effect of stress on VTA afferents.
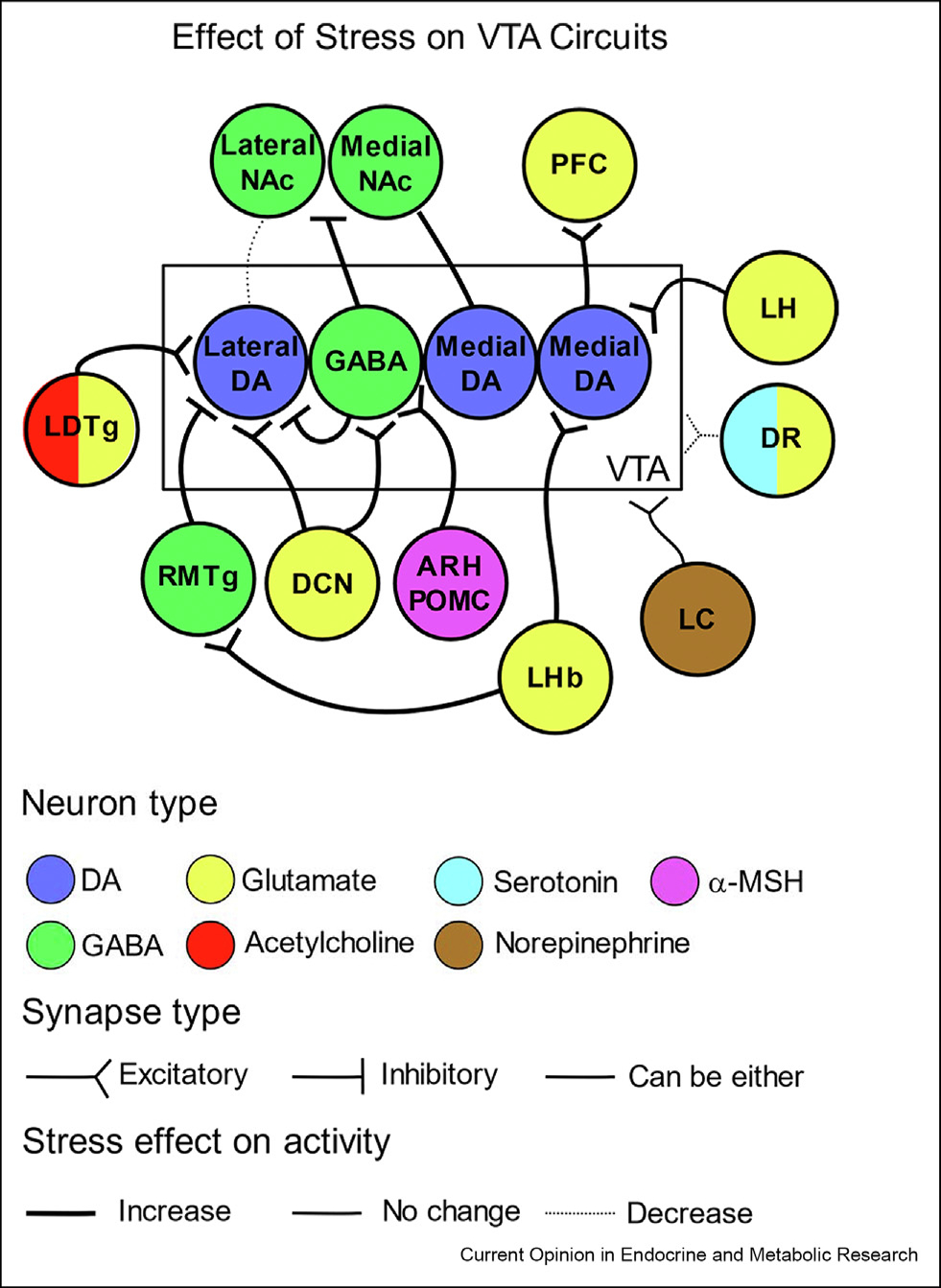
The change in activity on VTA-projecting afferents is represented by thick lines (increase in activity), thin lines (no change in activity), or dotted lines (decrease in activity). With the exception of the dorsal raphe (DR) and locus coeruleus (LC), synapses are shown on medial or lateral VTA DA neurons based on existing data. Brain regions are color-coded by neurotransmitter released onto the VTA. Excitatory synapses are drawn with a forked terminal, inhibitory synapses are drawn with a flat terminal, and synapses that can be excitatory or inhibitory are drawn with no terminal.
Serotonergic dorsal raphe (DR) neurons projecting to the VTA co-release glutamate and serotonin [90]. The firing rate of these neurons are decreased ex vivo in stress-susceptible animals and inhibiting/exciting these neurons bidirectionally controls stress susceptibility. Notably, the serotonin-mediated current was blocked by ketanserin, an antagonist of type a and c serotonin receptors. Future studies could examine how serotonin inputs from the DR impact the stress-induced depolarization of the chloride potential of VTA GABA neurons, as a typea serotonin receptor agonist can prevent this effect [91].
VTA-projecting locus coeruleus (LC) neurons exhibit enhanced ex vivo firing activity in stress-resilient mice [92]. Moreover, repeated optogenetic stimulation over 10 days of VTA-projecting LC neurons after chronic stress in stress-susceptible mice rescues social interaction, sucrose preference, and forced swim mobility, and increases firing rate of LC neurons like that observed in resilient mice. This repeated optogenetic stimulation of VTA-projecting LC neurons was found to induce homeostatic plasticity in NAc-projecting VTA neurons via increased potassium currents. Repeated co-infusion of α1 and β3 adrenergic receptor agonists into the VTA of stress-susceptible mice also reverses social avoidance, whereas co-infusion of α1 and β3 antagonists into the VTA before repeated stimulation of VTA-projecting LC neurons prevents the pro-resilient effect of this manipulation. These data suggest a possible role for LC inputs to the VTA in modulating stress-impaired behavior. However, another study found that subchronic variable stress, a stress model where rodents are exposed to footshocks, tail suspension, or restraint once a day for 6 days, does not affect VTA-projecting LC neuron activity, suggesting that selective stress paradigms recruit the LC [93].
Conclusion
Stress has a wide range of effects on neuronal activity within the VTA. Stress can excite or inhibit different VTA DA neuron subpopulations. By contrast, VTA GABA neurons tend to be excited after stress, although given their newly recognized heterogeneity, this principle needs to be systematically investigated in VTA GABA neuron subpopulations. An increasing body of ex vivo evidence has identified a variety of synaptic plasticity mechanisms for how stress modulates the activity of the VTA neurons. In this review, we propose that the diverse behavioral consequences of stress, including decreased reward anticipation, altered reward learning, and increased drug self-administration, stem from the actions of distinct neural subpopulations and the interaction of stress- and drug-induced plasticity. The remarkable advances the field has made in understanding the heterogeneity of VTA circuits and the impact of stress on ex vivo neural activity and plasticity raise several outstanding questions: 1. How do changes in electrophysiologic properties influence the in vivo responses of these neurons during distinct phases of reward processing? 2. How do different streams of information carried by VTA subpopulations get integrated during behavior? 3. How do different stressors (e.g., acute, chronic, active, passive) differentially impact reward circuits? 4. How does stress reduce reward-seeking yet facilitate drug self-administration? Is it via interactions of the plasticity induced by stress and drugs of abuse or mediated by distinct subpopulations? Closing these gaps in knowledge will provide important insight into more targeted treatment of stress-induced anhedonia and substance use.
Acknowledgments
We would like to thank the anonymous reviewers and Urszula Skupio for their helpful feedback on the manuscript.
Funding support
This work was supported by a grant from the NIMH to A.Z.H (R01 MH124998-01A1).
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
- 1.Watabe-Uchida M, Eshel N, Uchida N: Neural circuitry of reward prediction error. Annu Rev Neurosci 2017, 40:373–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouarab C, Thompson B, Polter AM: VTA GABA neurons at the interface of stress and reward. Front Neural Circ 2019, 13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliva I, Wanat MJ: Ventral tegmental area afferents and drug-dependent behaviors. Front Psychiatr 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein DS, McEwen B: Allostasis, homeostats, and the nature of stress. Stress 2002, 5:55–58. [DOI] [PubMed] [Google Scholar]
- 5.Yerkes RM, Dodson JD: The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 1908, 18:459–482. [Google Scholar]
- 6.Pelizza L, Ferrari A: Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatr 2009, 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller MB, et al. : Results of the DSM-IV mood disorders field trial. Am J Psychiatr 1995, 152:843–849. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association DSMTF. In Diagnostic and statistical manual of mental disorders : DSM-5. A. American Psychiatric, D. S. M. T. F. American Psychiatric Association. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 9.Sinha R: Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 2008, 1141:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanat MJ, Bonci A, Phillips PE: CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci 2013, 16:383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowes DC, et al. : Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat Commun 2021, 12:3539. *This paper identifies that VTA GABA neurons drive a 4 Hz LFP oscillation in the NAc which correlates with stress susceptibility. In addition, stress-induced plasticity in VTA GABA neuron activity drives impaired reward anticipation behavior.
- 12. Stelly CE, Tritley SC, Rafati Y, Wanat MJ: Acute stress enhances associative learning via dopamine signaling in the ventral lateral striatum. J Neurosci 2020, 40:4391–4400. *The authors reveal that after an acute stressor there is a paradoxical enhancement of reward learning, which may reflect an adaptive stress response.
- 13.Bogdan R, Pizzagalli DA: Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatr 2006, 60:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA: Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci 2011, 31:13246–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolova Y, Bogdan R, Pizzagalli DA: Perception of a naturalistic stressor interacts with 5-HTTLPR/rs25531 genotype and gender to impact reward responsiveness. Neuropsychobiology 2012, 65:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tovar-Díaz J, Pomrenze MB, Kan R, Pahlavan B, Morikawa H: Cooperative CRF and α1 adrenergic signaling in the VTA promotes NMDA plasticity and drives social stress enhancement of cocaine conditioning. Cell Rep 2018, 22:2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostroumov A, et al. : Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 2016, 92:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, DeBold JF, Miczek KA: Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 2017, 234:2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard MZ, DeBold JF, Miczek KA: Escalated cocaine “binges” in rats: enduring effects of social defeat stress or intra-VTA CRF. Psychopharmacology 2017, 234:2823–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polter AM, et al. : Constitutive activation of kappa opioid receptors at ventral tegmental area inhibitory synapses following acute stress. Elife 2017, 6:e23785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bale TL, Vale WW: CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004, 44:525–557. [DOI] [PubMed] [Google Scholar]
- 22.Herman JP, et al. : Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol 2003, 24:151–180. [DOI] [PubMed] [Google Scholar]
- 23.Daftary SS, Panksepp J, Dong Y, Saal DB: Stress-induced, glucocorticoid-dependent strengthening of glutamatergic synaptic transmission in midbrain dopamine neurons. Neurosci Lett 2009, 452:273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizoguchi A, et al. : Glucocorticoid receptor signaling in ventral tegmental area neurons increases the rewarding value of a high-fat diet in mice. Sci Rep 2021, 11:12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira AR, Reimer AE, Brandão ML: Mineralocorticoid receptors in the ventral tegmental area regulate dopamine efflux in the basolateral amygdala during the expression of conditioned fear. Psychoneuroendocrinology 2014, 43:114–125. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira AR, Reimer AE, Reis FMCV, Brandão ML: Conditioned fear response is modulated by a combined action of the hypothalamic–pituitary–adrenal axis and dopamine activity in the basolateral amygdala. Eur Neuropsychopharmacol 2013, 23:379–389. [DOI] [PubMed] [Google Scholar]
- 27.Tye SJ, Miller AD, Blaha CD: Differential corticosteroid receptor regulation of mesoaccumbens dopamine efflux during the peak and nadir of the circadian rhythm: a molecular equilibrium in the midbrain? Synapse 2009, 63:982–990. [DOI] [PubMed] [Google Scholar]
- 28.Rodaros D, Caruana DA, Amir S, Stewart J: Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 2007, 150:8–13. [DOI] [PubMed] [Google Scholar]
- 29.Dabrowska J, Martinon D, Moaddab M, Rainnie DG: Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol 2016, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinker JA, et al. : Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biol Psychiatr 2017, 81:930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieder TE, et al. : VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci 2014, 17:1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanat MJ, Hopf FW, Stuber GD, Phillips PEM, Bonci A: Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol 2008, 586:2157–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zalachoras I, et al. : Opposite effects of stress on effortful motivation in high and low anxiety are mediated by CRHR1 in the VTA. Sci Adv 2022, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David M, Serena B, Jeremy B, Madeline T, Bernard BW: CRF-receptor1 modulation of the dopamine projection to prelimbic cortex facilitates cognitive flexibility after acute and chronic stress. Neurobiol Stress 2022, 16:100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harlan BA, Becker HC, Woodward JJ, Riegel AC: Opposing actions of CRF-R1 and CB1 receptors on VTA-GABAergic plasticity following chronic exposure to ethanol. Neuropsychopharmacology 2018, 43:2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungless MA, et al. : Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 2003, 39:401–407. [DOI] [PubMed] [Google Scholar]
- 37.Knoll AT, Carlezon WA: Dynorphin, stress, and depression. Brain Res 2010, 1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muschamp JW, et al. : Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA 2014, 111:E1648–E1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margolis EB, Hjelmstad GO, Bonci A, Fields HL: Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 2003, 23:9981–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baimel C, Lau BK, Qiao M, Borgland SL: Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep 2017, 18:1346–1355. [DOI] [PubMed] [Google Scholar]
- 41.Margolis EB, Hjelmstad GO, Bonci A, Fields HL: Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol 2005, 93:3086–3093. [DOI] [PubMed] [Google Scholar]
- 42.Valentino RJ, Van Bockstaele E: Endogenous opioids: the downside of opposing stress. Neurobiol Stress 2015, 1:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu N, et al. : A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatr 2020, 25:1006–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toda C, Santoro A, Kim JD, Diano S: POMC neurons: from birth to death. Annu Rev Physiol 2017, 79:209–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N: Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 2012, 482:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anstrom KK, Miczek KA, Budygin EA: Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 2009, 161:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anstrom KK, Woodward DJ: Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 2005, 30:1832–1840. [DOI] [PubMed] [Google Scholar]
- 48.Morel C, et al. : Nicotinic receptors mediate stress-nicotine detrimental interplay via dopamine cells’ activity. Mol Psychiatr 2018, 23:1597–1605. [DOI] [PubMed] [Google Scholar]
- 49.Mirenowicz J, Schultz W: Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 1996, 379:449–451. [DOI] [PubMed] [Google Scholar]
- 50.Zhong W, Li Y, Feng Q, Luo M: Learning and stress shape the reward response patterns of serotonin neurons. J Neurosci 2017, 37:8863–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ungless MA, Magill PJ, Bolam JP: Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 2004, 303:2040–2042. [DOI] [PubMed] [Google Scholar]
- 52. Markovic T, et al. : Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nat Neurosci 2021, 24:1601–1613. *The authors report that an aversive stimulus in the form of pain can decrease VTA DA activity during reward behavior via increased inhibition from RMTg GABA neurons.
- 53.Valenti O, Gill KM, Grace AA: Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci 2012, 35:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valenti O, Lodge DJ, Grace AA: Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 2011, 31:4280–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zweifel LS, et al. : Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci 2011, 14:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Jong JW, et al. : A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron 2019, 101:133–151. e137. *This report reveals differential responses to aversive stimuli in VTA DA terminals in the lateral and medial NAc, with terminals in the lateral NAc inhibited then excited by the onset and offset of an aversive stimulus and with terminals in the medial NAc excited by the onset of an aversive stimulus.
- 57.Chaudhury D, et al. : Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lammel S, Ion DI, Roeper J, Malenka RC: Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011, 70:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabib S, Puglisi-Allegra S: The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 2012, 36:79–89. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, et al. : Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 2018, 97:434–449 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S: Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res 1991, 554:217–222. [DOI] [PubMed] [Google Scholar]
- 62.Zhong P, et al. : HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. Elife 2017, 7:e32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tye KM, et al. : Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stamatakis AM, Stuber GD: Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 2012, 15:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lammel S, et al. : Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012, 491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodge DJ, Grace AA: The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA 2006, 103:5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez SP, et al. : Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors. Nat Commun 2018, 9:4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baek SJ, Park JS, Kim J, Yamamoto Y, Tanaka-Yamamoto K: VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors. Elife 2022, 11:e72981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan L, Dou Y-N, Sun Y-G: Topography of reward and aversion encoding in the mesolimbic dopaminergic system. J Neurosci 2019, 39:6472–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cerniauskas I, et al. : Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 2019, 104:899–915. e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spyrka J, et al. : Early life stress-induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. Neurobiol Stress 2020, 13:100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouardouz M, Sastry BR: Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol 2000, 84:1414–1421. [DOI] [PubMed] [Google Scholar]
- 73.Nugent FS, Kauer JA: LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol 2008, 586:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graziane Nicholas M, Polter Abigail M, Briand Lisa A, Pierce RC, Kauer Julie A: Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron 2013, 77:942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lammel S, Lim BK, Malenka RC: Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76 Pt B:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wise RA JNr. Dopamine and reward: the anhedonia hypothesis 30 years on, vol. 14; 2008:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan KR, et al. : GABA neurons of the VTA drive conditioned place aversion. Neuron 2012, 73:1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou Z, et al. : A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron 2019, 103:473–488. e476. *This report identified a novel circuit through which an aversive stimulus can excite VTA GABA neurons.
- 79.Kauer JA, Malenka RC: Synaptic plasticity and addiction. Nat Rev Neurosci 2007, 8:844–858. [DOI] [PubMed] [Google Scholar]
- 80.Saal D, Dong Y, Bonci A, Malenka RC: Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003, 37:577–582. [DOI] [PubMed] [Google Scholar]
- 81.Dong Y, et al. : Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci U S A 2004, 101:14282–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudolph ML, Neve RL, Hammer RP Jr, Nikulina EM: Enhanced psychostimulant response, but not social avoidance, depends on GluA1 AMPA receptors in VTA dopamine neurons following intermittent social defeat stress in rats. Eur J Neurosci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abraham WC, Bear MF: Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 1996, 19:126–130. [DOI] [PubMed] [Google Scholar]
- 84.Lodge DJ, Grace AA: Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Therapeut 2005, 314:201. [DOI] [PubMed] [Google Scholar]
- 85.Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA: Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology 2011, 218:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang B, You Z-B, Rice KC, Wise RA: Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology 2007, 193:283–294. [DOI] [PubMed] [Google Scholar]
- 87.Nugent FS, Penick EC, Kauer JA: Opioids block long-term potentiation of inhibitory synapses. Nature 2007, 446:1086–1090. [DOI] [PubMed] [Google Scholar]
- 88.Guan Y-z, Ye J-H: Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving μ-opioid receptors. Neuropsychopharmacology 2010, 35:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niehaus JL, Murali M, Kauer JA: Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci 2010, 32:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou W-J, et al. : A discrete serotonergic circuit regulates vulnerability to social stress. Nat Commun 2020, 11:4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kimmey BA, Ostroumov A, Dani JA: 5-HT2A receptor activation normalizes stress-induced dysregulation of GABAergic signaling in the ventral tegmental area. Proc Natl Acad Sci USA 2019, 116:27028–27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, et al. : α1- and β3-adrenergic receptor–mediated mesolimbic homeostatic plasticity confers resilience to social stress in susceptible mice. Biol Psychiatr 2019, 85:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S, et al. : Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience 2018, 376:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]


