OBJECTIVES:
Severe COVID-19 is associated with exaggerated complement activation. We assessed the efficacy and safety of avdoralimab (an anti-C5aR1 mAb) in severe COVID-19.
DESIGN:
FOR COVID Elimination (FORCE) was a double-blind, placebo-controlled study.
SETTING:
Twelve clinical sites in France (ICU and general hospitals).
PATIENTS:
Patients receiving greater than or equal to 5 L oxygen/min to maintain Spo2 greater than 93% (World Health Organization scale ≥ 5). Patients received conventional oxygen therapy or high-flow oxygen (HFO)/noninvasive ventilation (NIV) in cohort 1; HFO, NIV, or invasive mechanical ventilation (IMV) in cohort 2; and IMV in cohort 3.
INTERVENTIONS:
Patients were randomly assigned, in a 1:1 ratio, to receive avdoralimab or placebo. The primary outcome was clinical status on the World Health Organization ordinal scale at days 14 and 28 for cohorts 1 and 3, and the number of ventilator-free days at day 28 (VFD28) for cohort 2.
MEASUREMENTS AND MAIN RESULTS:
We randomized 207 patients: 99 in cohort 1, 49 in cohort 2, and 59 in cohort 3. During hospitalization, 95% of patients received glucocorticoids. Avdoralimab did not improve World Health Organization clinical scale score on days 14 and 28 (between-group difference on day 28 of –0.26 (95% CI, –1.2 to 0.7; p = 0.7) in cohort 1 and –0.28 (95% CI, –1.8 to 1.2; p = 0.6) in cohort 3). Avdoralimab did not improve VFD28 in cohort 2 (between-group difference of –6.3 (95% CI, –13.2 to 0.7; p = 0.96) or secondary outcomes in any cohort. No subgroup of interest was identified.
CONCLUSIONS:
In this randomized trial in hospitalized patients with severe COVID-19 pneumonia, avdoralimab did not significantly improve clinical status at days 14 and 28 (funded by Innate Pharma, ClinicalTrials.gov number, NCT04371367).
Keywords: avdoralimab, complement, COVID-19, inflammation, sepsis
KEY POINTS.
Question: We assessed the efficacy and safety of avdoralimab (complement blockade with an anti-C5aR1 mAb) in patients with severe COVID-19.
Findings: In this double-blind, placebo-controlled study, C5aR1 blockade in severe COVID-19 did not improve clinical status at days 14 and 28.
Meaning: These results do not support further evaluations of complement blockade for severe COVID-19 treatment in the context of actual standard-of-care.
Since its emergence in China in 2019, the original strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants have infected ~260 million people globally, and over 5 million people worldwide had died from COVID-19 by the end of 2021 (1). With the progression of the pandemic, very few treatment options have proved effective, and COVID-19 continues to be a major public health problem. In 10–20% of hospitalized patients, transfer to an ICU is required, due to acute respiratory distress syndrome, requiring high-flow oxygen (HFO), noninvasive ventilation (NIV), or invasive mechanical ventilation (IMV) (2, 3). Severe COVID-19 is characterized by overt inflammation of the lungs in response to the viral infection (4–6). No antiviral treatment has yet displayed strong clinical efficacy (7). Immunomodulatory drugs known to delay hyperinflammation—principally dexamethasone (8), but also, to a lesser extent, tocilizumab (9, 10), anakinra (11), and Janus Kinase (JAK) inhibitors (12, 13)—have been shown to be effective, to some extent. However, despite substantial changes to the treatments prescribed, no significant improvements in ICU mortality rates between the first wave and the next two waves have been reported (14).
A link between exaggerated activation of the complement cascade and the initiation, maintenance, and amplification of inflammation has been reported in severe COVID-19 (15–17). Furthermore, systemic activation of the complement cascade may, indeed, lead, via C5a, to the recruitment of neutrophils to the pulmonary endothelium and their activation, resulting in endothelial cell damage and subsequent lung injury (18, 19). Consistent with these results, we reported an association of higher levels of soluble C5a with more severe COVID-19 in a longitudinal study assessing the immune responses of 82 individuals (20). We also found that C5b9 accumulated in the lung sections of patients with COVID-19. Accordingly, small proof-of-concept studies have suggested that inhibiting the C5a-C5aR axis might improve survival and reduce hypoxia in patients with severe COVID-19 (21, 22).
Avdoralimab is a monoclonal antibody against C5aR1 that inhibits the signaling of C5a via its receptor. In FOR COVID Elimination (FORCE), a double-blind, randomized, placebo-controlled study, we evaluated the safety and efficacy of avdoralimab in patients with severe COVID-19 pneumonia defined as a requirement for oxygen therapy greater than or equal to 5 L/min to maintain blood oxygen saturation levels (Spo2) greater than 93%.
MATERIALS AND METHODS
Trial Oversight
FORCE was a double-blind, placebo-controlled phase II clinical trial conducted at 12 clinical sites in France (full list in the Supplementary Appendix, http://links.lww.com/CCM/H213), and coordinated by Assistance Publique des Hôpitaux de Marseille. The trial protocol was approved by the French National Agency for the Safety of Medicines and Health Products (MEDAECNAT-2020-04-00043_2020-001686-36), and the competent ethics committee (Comité de Protection des Personnes Ouest II CPP #2020/34/Covid-19, EudraCT2020-001686-36) and was overseen by an independent data and safety monitoring board. Procedures were performed in accordance with ethical standards (institutional and national) for human experimentation and the 1975 Helsinki Declaration. Data were analyzed by independent statisticians at the International Drug Development Institute. Written informed consent was obtained from all patients or their legal representatives (if the patient was unable to provide consent). The trial was registered on ClinicalTrials.gov (NCT04371367).
Trial Population
We enrolled adults between 18 and 80 years old in this trial. Hospitalized patients were eligible for inclusion if they had severe COVID-19 pneumonia requiring oxygen therapy greater than or equal to 5 L/min to maintain blood Spo2 greater than 93% (World Health Organization [WHO] ordinal scale ≥ 5; Table S1, http://links.lww.com/CCM/H213). COVID-19 diagnosis was confirmed by a positive reverse transcription-polymerase chain reaction (PCR) assay for SARS-CoV-2, or, for cohort 2, on the basis of CT imaging findings typical of COVID-19 (peripheral ground glass opacities).
Cohort 1 consisted of patients requiring conventional oxygen therapy via masks or nasal prongs at greater than or equal to 5 L/min (WHO score 5) and patients on HFO (WHO score 6). Cohort 2 contained patients in the ICU requiring HFO, NIV, or IMV (WHO scores, 6–9). Cohort 3 contained patients on IMV for less than 72 hours, with a ratio of the Po2 to the Fio2 (Pao2/Fio2 [P/F]) of 60 to 200 mm Hg (WHO scores, 7–9).
Initially, the trial was designed to include two cohorts. Cohort 1 consisted of patients admitted to a general hospital and requiring conventional oxygen therapy via masks or nasal prongs at greater than or equal to 5 L/min (WHO score, 5), and cohort 2 contained patients admitted to the ICU requiring HFO, NIV, or IMV (WHO scores, 6–9). In the face of difficulties enrolling patients in cohort 1 (due to patients requiring rapid transfer to the ICU for HFO treatment), the trial was amended without breaking blinding in August 2020. In this new design, once cohort 2 was closed for enrollment, patients receiving HFO were enrolled in cohort 1 (WHO scores, 5–6). Patients on IMV for less than 72 hours with a Pao2/Fio2 ratio of 60 to 200 mm Hg were enrolled in cohort 3 (WHO scores, 7–9).
For all cohorts, the exclusion criteria included being pregnant or breastfeeding, being enrolled in another clinical trial, having active bacterial or fungal sepsis at the time of enrollment, or having contraindications preventing ICU admission. After trial amendment, for cohorts 1 and 3, the exclusion criteria included receiving extracorporeal membrane oxygenation, having chronic pulmonary disease, or requiring immediate renal support therapy. Patients were allowed to receive glucocorticoids, including dexamethasone (12). However, concomitant therapy with immunomodulatory or antiviral agents (such as tocilizumab, anakinra, or hydroxychloroquine) was prohibited. The use of antibiotic prophylaxis was not specifically recommended.
Randomization and Treatment
Eligible patients were randomly assigned, in a 1:1 ratio, to the avdoralimab or placebo arms, via a web-based case-report form system. Randomization was performed in blocks of four, with stratification by trial site and type of admission (ICU or general hospital). Patients received avdoralimab (500-mg loading dose followed by a 200-mg maintenance dose) or placebo (normal saline) administered intravenously every 48 hours until oxygen therapy was no longer needed, and for a maximum of 14 days (eight injections). Treatment could be discontinued if an adverse event potentially linked to the treatment occurred. Only trial-site pharmacists were aware of the treatment assigned to the patient. All other trial-site personnel and patients were blind to treatment assignment during the study.
Outcome Measures
For cohorts 1 and 3, the primary outcome was clinical status improvement on days 14 and 28, assessed with the WHO ordinal scale. For patients in cohort 2, the primary outcome was the number of ventilator-free days at day 28 (VFD28). VFD28 was assessed 28 days after enrollment and was defined as the number of days on which the patients were alive and had been completely weaned off IMV for at least 48 hours. Patients who had died by day 28 were considered to have had zero ventilator-free days (23).
The secondary outcome measures were clinical status improvement on days 14 and 28 according to the WHO ordinal scale (cohort 2), ventilator-free days at day 14 (VFD14) and VFD28 for ICU patients, Sequential Organ Failure Assessment (SOFA) score at day 14 in ICU patients, the occurrence of adverse events, the occurrence of secondary bacterial or fungal sepsis (24), the rate of patient discharge alive from the hospital or from the ICU before day 14 (WHO score < 4 or < 6, respectively), and mortality at day 28.
Statistical Analysis
The statistical analysis was based on Gaussian tests comparing the mean clinical improvement assessed with the WHO score ordinal scale (cohorts 1 and 3) and mean VFD28 (cohort 2). For cohort 1, we calculated that a sample size of 90 patients would provide a power of more than 76% for detecting a 1.0-point difference on day 14 and a power of more than 88% for detecting a 1.2-point difference between treatment arms on day 28. For cohort 2, a sample size of 48 patients would provide a power of more than 96% for detecting a clinically significant improvement of at least 2 days in the primary outcome (VFD28) with a 5% type I error (one-tailed test). For cohort 3, a sample of 60 patients would provide the trial with a power of more than 80% for detecting a 1.3-point difference at day 14 and a power of more than 89% for detecting a 1.5-point difference between treatment arms at day 28. A fallback procedure was used to control for familywise type I error after multiple testing (25).
The intention-to-treat population (ITT) included all patients randomized and assigned to a treatment arm. In cohort 1, the modified-ITT (m-ITT) population included only the patients enrolled after protocol amendment. The per-protocol (PP) population was based on the treatment effectively received; patients not meeting the inclusion criteria were excluded. The population for the safety analysis included all patients receiving at least one dose of avdoralimab or placebo.
Post hoc exploratory analyses were performed for factors believed to influence COVID-19 outcomes. Logistic regression models with odds ratios were used to evaluate the impact of known prognostic factors for COVID-19 on WHO ordinal scale score at day 14 (≤ 4 vs ≥ 5) and mortality at day 28. In both models, treatment arm was forced into the model, to evaluate the impact of treatment on outcome.
The statistical analyses were performed with the SAS software Version 9.4 and the RStudio software version 1.1.456. Sample size was calculated with the East 6 software (Cytel). Results are expressed as means (sd).
Supplementary methods (selection of avdoralimab dose, additional statistical analyses, and exploratory laboratory analysis) are detailed in Appendix 2 (http://links.lww.com/CCM/H213). The full protocol and statistical analysis plan detailing the statistical approach are available in Appendix 5 (http://links.lww.com/CCM/H213) (protocol details).
RESULTS
Patient Characteristics
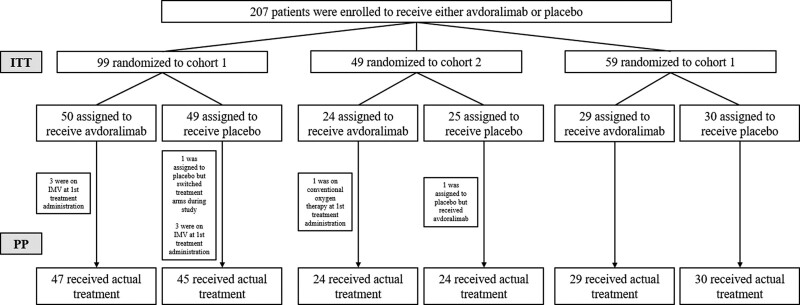
Between May 2020 and January 2021, 207 patients were randomized and included in the ITT population: 99 patients in cohort 1 (50 and 49 in the avdoralimab and placebo groups, respectively); 49 patients in cohort 2 (24 and 25, respectively); and 59 patients in cohort 3 (29 and 30, respectively). The ITT and PP populations are shown in Figure 1. Reasons for withdrawal from the study are described in Table S2 (http://links.lww.com/CCM/H213).
Figure 1.
Flowchart. In cohort 1, 50 patients were assigned to receive avdoralimab (intention-to-treat [ITT] population), and 47 (94%) received the treatment as assigned (per-protocol [PP] population): three were on invasive mechanical ventilation (IMV) at first treatment administration. In cohort 1, 49 patients were assigned to receive placebo (ITT population), and 45 (92%) received placebo as assigned (PP population): one was assigned to placebo but switched treatment arms during the study, and three were on IMV at first treatment administration. In cohort 2, 24 patients were assigned to receive avdoralimab (ITT population), and 23 received the treatment as assigned. One patient was on conventional oxygen therapy at first treatment administration. In cohort 2, 25 patients were assigned to receive placebo (ITT population), and 24 (96%) received placebo as assigned: one was assigned to placebo but received avdoralimab. As this patient received only avdoralimab, he was switched to the avdoralimab group in the PP population. In cohort 3, 100% of patients received their assigned treatment in either arm; 29 patients received avdoralimab, and 30 patients received placebo (ITT and PP populations).
Baseline demographic data and medical history were largely similar between cohorts and treatment groups (Table 1). Mean patient age was higher for the avdoralimab-treated patients in cohort 3 (69.1 yr [7.5]), the mean age across cohorts being 63.7 years (10.4). Most of the patients enrolled were male (71%; n = 147/207) (Table S3, http://links.lww.com/CCM/H213—for the characteristics of the total population at baseline). Most patients had at least one risk factor at baseline, mostly hypertension (51%, n = 105/207), obesity (45%, n = 93/207), or type 2 diabetes (36%, n = 75/207). In cohort 2, obesity was more prevalent among the patients of the placebo group (64%, n = 16 vs 33%, n = 8). A larger number of patients in the avdoralimab group than those in the placebo group had comorbidities: cancer (21%, n = 5 vs 4%, n = 1) in cohort 2, cardiovascular disease in cohort 2 (33%, n = 8 vs 16%, n = 4) and cohort 3 (28%, n = 8 vs 17%, n = 5), and chronic obstructive pulmonary disease (21%, n = 6 vs 7%, n = 2) in cohort 3.
TABLE 1.
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| Demographic data | Avdoralimab, n = 50 | Placebo, n = 49 | Avdoralimab, n = 24 | Placebo, n = 25 | Avdoralimab, n = 29 | Placebo, n = 30 |
| Age, yr | 63.2 (10.4) | 60.8 (12.5) | 65.0 (7.8) | 63.3 (9) | 69.1 (7.5) | 63.6 (10.6) |
| Sex—M (n [%]) | 36 (72) | 30 (61) | 18 (75) | 19 (76) | 21 (72) | 23 (77) |
| BMI (kg/m2) | 29.8 (5.9) | 30.3 (5.7) | 28.9 (5.7) | 31.2 (6.0) | 31.1 (4.2) | 30.3 (4.7) |
| Risk factors | ||||||
| Obesity (n [%]) | 25 (50) | 19 (39) | 8 (33) | 16 (64) | 12 (41) | 13 (43) |
| Hypertension (n [%]) | 25 (50) | 26 (53) | 9 (38) | 13 (52) | 19 (66) | 13 (43) |
| Diabetes (n [%]) | 16 (32) | 18 (37) | 10 (42) | 10 (40) | 14 (48) | 7 (23) |
| Comorbidities (chronic diseases) | ||||||
| Cardiovascular disease (n [%]) | 13 (26) | 12 (25) | 8 (33) | 4 (16) | 8 (28) | 5 (17) |
| Chronic obstructive pulmonary disease (n [%]) | 1 (2) | 3 (6) | 1 (4) | 3 (12) | 6 (21) | 2 (7) |
| Chronic kidney disease (n [%]) | 1 (2) | 3 (6) | 2 (8) | 2 (8) | 2 (7) | 1 (3) |
| Cancer (n [%]) | 7 (14) | 5 (10) | 5 (21) | 1 (4) | 2 (7) | 2 (7) |
| COVID-19 diagnosis | ||||||
| Positive severe acute respiratory syndrome coronavirus-2 reverse transcription polymerase chain reaction (n [%])c | 50 (100) | 49 (100) | 23 (96) | 24 (96) | 29 (100) | 30 (100) |
| Viral load at inclusion (Ct)d | 27.9 (6.6) | 23.8 (8.1) | 23.3 (4.6) | 24.4 (4.2) | 27.1 (5.6) | 26.6 (6) |
| Time from first signs to inclusion (d) | 9.2 (3.3) | 9.8 (2.9) | 10.1 (3.9) | 10.2 (4.1) | 10.6 (4.5) | 10.7 (3.2) |
| Time from hospitalization to inclusion (d) | 2.1 (1.7) | 2.4 (2.5) | 2 (1.6) | 1.4 (1.2) | 2.3 (1.6) | 2.8 (1.8) |
| COVID-19 severity at inclusion | ||||||
| World Health Organization Scale (n [%]) | ||||||
| 5: Hospitalized, oxygen by mask or nasal prongs | 8 (16) | 10 (20) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| 6: Hospitalized, oxygen by NIV or HFO | 40 (80)e | 36 (74) | 13 (54) | 15 (60) | 0 (0) | 0 (0) |
| 7: IMV and P/F ratio ≥ 150 | 0 (0) | 1 (2) | 5 (21) | 2 (8) | 3 (10) | 4 (13) |
| 8: IMV and P/F ratio < 150 or vasopressors | 0 (0) | 0 (0) | 4 (17) | 4 (16) | 15 (52) | 14 (47) |
| 9: IMV and P/F ratio < 150 and vasopressors, RRT, or extracorporeal membrane oxygenation | 2 (4) | 2 (4) | 1 (4) | 4 (16) | 11 (38) | 12 (40) |
| Sequential Organ Failure Assessment | 3.8 (1.3) | 4.0 (1.6) | 5.2 (2.2) | 5.7 (3.0) | 7.1 (2.7) | 6.7 (2.3) |
| P/F ratio—ICU patients (HFO/NIV or IMV) | 119 (52) | 118 (64) | 133 (62) | 131 (57) | 127 (56) | 130 (48) |
| Positive end-expiratory pressure (cm H2O)—IMV patients | — | — | 12.6 (1.9) | 12.4 (3.8) | 12.8 (2.8) | 12 (2.5) |
| Tidal volume (mL)—IMV patients | — | — | 422 (17) | 417 (52) | 410 (53) | 418 (51) |
| Lesions on CT-scans, n (%) | ||||||
| Limited | 3 (6) | 3 (6) | 3 (13) | 1 (4) | 1 (3) | 0 (0) |
| Moderate | 20 (40) | 18 (37) | 2 (8) | 4 (16) | 5 (17) | 8 (27) |
| Severe | 10 (20) | 18 (37) | 8 (33) | 7 (28) | 6 (21) | 6 (20) |
| CT scan not done | 17 (34) | 10 (20) | 11 (46) | 13 (52) | 17 (59) | 16 (53) |
| Concomitant drugs, n (%) | ||||||
| Antibiotics | 15 (30) | 14 (29) | 6 (24) | 7 (29) | 15 (52) | 18 (60) |
| Dexamethasone | 41 (82) | 42 (86) | 22 (88) | 18 (75) | 28 (97) | 25 (83) |
| Methylprednisolone | 15 (30) | 11 (23) | 3 (13) | 5 (20) | 5 (17) | 10 (33) |
| Number of perfusions (avdoralimab or placebo) | 5.7 (2.1) | 5.3 (2.2) | 6.1 (2.3) | 6 (2.3) | 6.1 (2.4) | 6.8 (2) |
HFO = high-flow oxygen, IMV = invasive mechanical ventilation, NIV = noninvasive ventilation, P/F = Pao2/Fio2.
Plus-minus values are means ± sd.
Baseline was defined as the last known observation before the administration of avdoralimab or placebo on day 1.
Two patients in cohort 2 were not tested by polymerase chain reaction. For these patients, diagnosis was confirmed by lung CT-scan
Viral load was assessed in a total of 88 patients. In cohort 1: n = 15 (avdoralimab arm) and n = 14 (placebo arm). In cohort 2: n = 11 (avdoralimab arm) and n = 14 (placebo arm). In cohort 3: n = 17 (avdoralimab arm) and n = 17 (placebo arm).
One patient included in this category was on IMV at the time of first treatment administration but was listed as having a World Health Organization score of 6 at inclusion.
Most patients (95%, n = 196) received glucocorticoids (including dexamethasone and methylprednisolone) during the course of the trial (Table 1; and Table S3, http://links.lww.com/CCM/H213).
Disease characteristics at baseline were also well-balanced between the treatment arms (Table S4, http://links.lww.com/CCM/H213). All but two patients had SARS-CoV-2 infection confirmed by nasopharyngeal PCR test. The two remaining patients were enrolled in cohort 2, and their COVID-19 diagnosis was confirmed by lung CT scan. The mean (±sd) time from the onset of COVID-19 symptoms to enrollment was 9.8 days (±3.8 d) in the avdoralimab-treated groups and 10.1 (±3.3) in the placebo-treated groups. Disease severity, as assessed by SOFA, Simplified Acute Physiology Score 2 scores and P/F ratios (Table 1), and mean number of perfusions (Table 1; details in Table S11, http://links.lww.com/CCM/H213) were similar for all treatment arms in all three cohorts (Table 1).
Primary Outcome
In cohorts 1 and 3, there was no significant difference in clinical improvement on the WHO ordinal scale between patients in the avdoralimab and placebo groups, on days 14 and 28 (Table 2). On day 28, the between-group difference in mean WHO scale improvement between the groups (avdoralimab vs placebo, so negative results mean favor placebo and positive results favor avdoralimab) was –0.3 (95% CI, –1.2 to 0.7; p = 0.7) in cohort 1; and –0.3 (95% CI, –1.8 to 1.2; p = 0.6) in cohort 3. The data for day 14 are shown in Table 2. Similar results were obtained for the m-ITT and PP populations in all cohorts (Tables S5 and S6, http://links.lww.com/CCM/H213).
TABLE 2.
Primary and Secondary Efficacy Outcomes (Intention-to-Treat Population Population) a
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| Outcomes | Avdoralimab, n = 50 | Placebo, n = 49 | Avdoralimab, n = 24 | Placebo, n = 25 | Avdoralimab, n = 29 | Placebo, n = 30 |
| World Health Organization scale score improvement | ||||||
| D14 | 1.2 (2.3) | 1.4 (2.1) | 0.7 (2.6) | 1.4 (1.7) | 1.2 (2.5) | 1.4 (1.9) |
| Difference of the means (avdoralimab vs placebo) (95% CI)b | –0.3 (–1.1 to 0.6) | –0.7 (–2.0 to 0.6) | –0.2 (–1.3 to 1.0) | |||
| p | 0.71 | 0.86 | 0.61 | |||
| D28 | 1.7 (2.8) | 1.9 (2.1) | 0.5 (3.1) | 2.7 (2.1) | 1.7 (3.0) | 1.9 (2.6) |
| Difference of the means (avdoralimab vs placebo) (95% CI)b | –0.3 (–1.2 to 0.7) | –2.2 (–3.7 to –0.6) | –0.3 (–1.8 to 1.2) | |||
| pc | 0.70 | 0.99 | 0.65 | |||
| VFD | ||||||
| VFD14 | NA | NA | 6.9 (6.7) | 7.1 (6.4) | 3.2 (4.4) | 2.4 (4.2) |
| Difference of the means (avdoralimab vs placebo) (95% CI)b | — | — | –0.3 (–4.0 to 3.5) | 0.8 (–1.4 to 3.1) | ||
| p c | — | — | 0.55 | 0.23 | ||
| VFD28 | NA | NA | 12.1 (13.6) | 18.4 (10.4) | 8.8 (11.1) | 7.7 (10.3) |
| Difference of the means (avdoralimab vs placebo) (95% CI)b | — | — | –6.3 (–13.2 to 0.7) | 1.0 (–4.5 to 6.6) | ||
| p c | — | — | 0.96 | 0.36 | ||
| Mortality at D28, n (%) | 6 (12) | 3 (6) | 4 (17) | 2 (8) | 10 (35) | 7 (23) |
| p c | 0.32 | 0.36 | 0.35 | |||
| AEs | ||||||
| Sepsis | 6 (12) | 8 (16) | 6 (24) | 7 (29) | 10 (35) | 10 (33) |
| Related AE | 9 (18) | 13 (27) | 8 (32) | 9 (38) | 7 (24) | 9 (30) |
| Serious AE | 18 (36) | 13 (27) | 13 (52) | 11 (46) | 19 (66) | 20 (67) |
AE = adverse event, NA = not applicable, VFD = ventilator-free day.
Plus-minus values are means ± sd.
A negative result favors placebo. A positive result favors avdoralimab.
In a one-tailed Student t test (avdoralimab-placebo > 0).
For cohort 2, mean (±sd) VFD28 was 12.1 (13.6) for the avdoralimab group and 18.4 (10.4) for the placebo group, with a between-group difference of –6.3 days (95% CI, –13.2 to 0.7; p = 0.96) (Table 2).
Key Secondary and Safety Outcomes
Avdoralimab was not effective for any of the secondary outcomes (Table 2; and Tables S5 and S6, http://links.lww.com/CCM/H213, for the m-ITT and PP populations, respectively). In cohort 2, no difference in clinical improvement on the WHO scale was observed between patients in the avdoralimab and placebo groups on days 14 and 28. On day 28, the between-group difference was –2.2 days (95% CI, –3.7 to –0.6; p = 0.99 in a one-tailed Student t test). In cohort 3, VFD14 and VFD28 were similar between the avdoralimab and placebo groups. Mean (sd) VFD28 was 8.8 (11.1) with avdoralimab and 7.7 (10.3) with placebo, with a between-group difference of 1 day (95% CI, –4.5 to 6.6; p = 0.36 in a one-tailed Student t test). Data for VFD14 are detailed in Table 2. Mortality rates at day 28 were higher, although not significantly, in the patients treated with avdoralimab than in those treated with placebo (19.4%, n = 20 vs 11.5%, n = 12, p = 0.12) (Table 2; and Tables S5 and S6 and Fig. S1, http://links.lww.com/CCM/H213).
SOFA score improvement at day 14 and the percentage of patients discharged from the hospital or from the ICU at days 7, 14, 21, and 28 were not improved by avdoralimab. In cohort 2, cumulative rates of discharge from hospital and the ICU were higher in the placebo group (Table S7, http://links.lww.com/CCM/H213).
Serious adverse events were reported in 48% (n = 50) and 43% (n = 44) of patients treated with avdoralimab and placebo, respectively. Sepsis was reported in 21% (n = 22) and 24% (n = 25) of patients treated with avdoralimab and placebo, respectively. Fatal events occurred in 27% (n = 28) of patients receiving avdoralimab and 16% (n = 16) of those receiving placebo, over all cohorts. Similar percentages of patients in each cohort had adverse events or serious adverse events (Table S8, http://links.lww.com/CCM/H213). The most commonly reported cause of death was COVID-19 pneumonia or COVID-19-related complications (Table S9, http://links.lww.com/CCM/H213).
Post Hoc Exploratory Analyses
We accounted for factors believed to influence COVID-19 outcomes, by performing a multivariable logistic regression in which we forced the treatment arm variable into the model. Age, diabetes, comorbidities, sepsis, and IMV were associated with a worse respiratory prognosis, whereas obesity was associated with a better change in WHO score at day 14. Age and comorbidities were independently associated with the occurrence of death (Table 3). Subgroup analysis identified no patient subgroup potentially benefiting from avdoralimab treatment (Figs. S2 and S3, http://links.lww.com/CCM/H213). The change in inflammation biomarkers, including interleukin-6, C5a, and C-reactive protein (CRP), did not differ between the avdoralimab and placebo groups over time. No delay in viral clearance or in seroconversion time was observed in patients treated with avdoralimab (Table S10, http://links.lww.com/CCM/H213).
TABLE 3.
Factors Independently Associated With World Health Organization Score at D14 and D28 Mortality (Intention-to-Treat Population Population)
| World Health Organization Score at Day 14 | |
|---|---|
| Covariates | ORa (95% CI) |
| Treatment (avdoralimab vs placebo) | 0.86 (0.42–1.77) |
| Obesity—Yes vs Nob | 0.45 (0.21–0.98) |
| Age (yr)—(65–79) vs (36–65) | 2.30 (1.07–4.97) |
| Comorbidity—Yes vs Nob,c | 2.78 (1.20–6.44) |
| Diabetes—Yes vs Nob | 2.85 (1.28–6.37) |
| Ventilation—Yes vs No | 6.34 (2.82–14.23) |
| Sepsis—Yes vs No | 14.01 (3.02–65.13) |
| Deaths by Day 28 | |
| Covariates | ORa (95% CI) |
| Treatment—avdoralimab vs placebo | 1.91 (0.83–4.40) |
| Age (yr)—(65–79) vs (36–65) | 3.47 (1.21–9.98) |
| Comorbidity—Yes vs Nob,c | 4.71 (1.90–11.65) |
OR = odds ratio.
Logistic regression model with treatment forced into the model. Candidate variables were selected as covariates with the stepwise procedure.
Missing data were considered as “No” for these covariates.
Comorbidities refer to chronic diseases (cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and cirrhosis or cancer).
DISCUSSION
There is clinical and exploratory evidence that exposure to SARS-CoV-2 triggers an overactivation of the complement system, partly via binding of the lectin and alternative pathway recognition molecules to both the SARS-CoV-2 N and S proteins (26, 27). Amplification of the complement response results in the overproduction of complement molecules, such as the C3a and C5a anaphylatoxins. C5a is a powerful anaphylatoxin that binds to C5aR1 on myeloid cells (including polymorphonuclear neutrophils, monocytes, and macrophages) and recruits them to the site of injury, the lungs in the case of COVID-19. Overactivation of the C5a-C5aR1 axis was found to be associated with hyperinflammation and endothelial lung injury in patients with severe COVID-19 during the first wave of the epidemic in France (20).
The results of the FORCE study (performed during the second wave in France) confirm that patients with severe COVID-19 have high serum concentrations of C5a, but C5aR1 blockade with avdoralimab provided no clinical benefit. It should be noted that the patients enrolled in FORCE had a different inflammatory profile, with lower CRP levels, than the patients observed in the first wave of the COVID-19 epidemic (Fig. S5, http://links.lww.com/CCM/H213). The appearance of SARS-CoV-2 variants and significant changes in the treatments prescribed, mainly corticosteroids, may have affected the inflammatory profiles of these patients. Consistent with this hypothesis, treatment with dexamethasone inhibited the proinflammatory action of C5a in a mouse model of acute lung injury (data not shown).
The results of the FORCE study also suggest that patients treated with avdoralimab may have a worse prognosis in terms of clinical respiratory progression and mortality. The patients in the avdoralimab group were slightly older and presented with a slightly larger number of comorbidities (including cardiovascular disease and cancer) with a well-established impact on COVID-19 prognosis (28). The observed effects do not seem to be related to drug toxicity, as the number of adverse events and serious adverse events, including sepsis, was similar in the avdoralimab- and placebo-treated patients. Furthermore, higher drug concentrations in the bloodstream were not found to be associated with worse disease progression (Fig. S4, http://links.lww.com/CCM/H213). Most of the deaths reported during this trial were due to COVID-19 complications (Table S9, http://links.lww.com/CCM/H213).
However, we cannot exclude the possibility that avdoralimab treatment triggers immunosuppression in patients, especially when combined with dexamethasone. This combination of treatments may have had a negative impact on disease outcomes, by favoring viral persistence (29) and the occurrence of secondary bacterial or fungal infections, as shown for other immunomodulatory drugs (30). We did not detect persistent viremia or delays in seroconversion time in the patients from the avdoralimab group; however, these results are not sufficient in themselves to attest to the absence of more severe immunosuppression in these patients.
CONCLUSION
The FORCE trial results show that, in the context of severe COVID-19 pneumonia, avdoralimab use does not improve clinical status, over placebo, at days 14 and 28. These results do not encourage further evaluation of this drug for COVID-19 treatment.
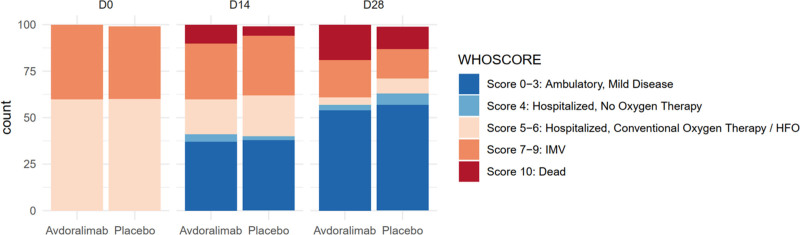
Figure 2.
Changes in clinical status according to the World Health Organization (WHO) ordinal scale (per-protocol [PP]-actual treatment). Categories on the WHO ordinal scale range from 0 to 10, with higher categories indicating a worse condition. Category 0 indicates that the patient was uninfected; 1, viral RNA but asymptomatic; 2, symptomatic, with no limitations on activities; 3, symptomatic needing assistance; 4, hospitalized with no need for oxygen therapy; 5, hospitalized receiving oxygen by mask or nasal prongs; 6, hospitalized receiving oxygen by noninvasive ventilation or high-flow oxygen; 7, intubated and receiving invasive mechanical ventilation (IMV) with Pao2/Fio2 ≥ 150; 8, receiving IMV with Pao2/Fio2 < 150 or vasopressors; 9, receiving IMV with Pao2/Fio2 < 150 and vasopressors, dialysis or extracorporeal membrane oxygenation; and 10, dead.
ACKNOWLEDGMENTS
We thank all our patients, supporters, and families for their confidence in our work. We thank all the healthcare workers involved in the analysis, diagnosis, and treatment of patients at Assistance Publique des Hôpitaux de Marseille and Hôpital Laveran. The E.V. laboratory at CIML and Assistance-Publique des Hôpitaux de Marseille is supported by funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (TILC, grant agreement no. 694502 and MInfla-TILC, grant agreement no. 875102 - MInfla-Tilc), the Agence Nationale de la Recherche, MSDAvenir, Innate Pharma, and institutional grants awarded to the CIML (INSERM, CNRS, and Aix-Marseille University) and Marseille Immunopole.
Supplementary Material
Footnotes
*See also p. 1840.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Carvelli, Karakunnel, Boyer-Chammard, Schleinitz, and Vivier initiated and designed the research. Drs. Carvelli and Fares wrote the article with the help of other coauthors. Drs. Piperoglou, Vely, Demaria, and Batista performed the experiments and analyzed and/or interpreted results. Drs. Batista, Rotolo, Viotti performed statistical analysis. Drs. Carvelli, Meziani, Dellamonica, Cordier, Allardet-Servent, Fraisse, Velly, Barbar, Lehingue, Guervilly, Desgrouas, Camou, Le Dault, Carles, and Schleinitz were in charge of patient care and contributed to the discussion of the results.
Supported, in part, by Innate Pharma.
Dr. Carvelli received support for article research from Innate Pharma. Drs. Carvelli, Allardet-Servent, Barbar, Desgrouas, Camou, Piperoglou, Viotti, Boyer-Chammard, Lacombe, Le Dault, Schleinitz, and Vivier disclosed the off-label product use of avdoralimab. Dr. Guervilly received funding from Xenios FMC. Dr. Demaria’s institution received funding from BPI. Drs. Demaria, Karakunnel, Fares, Batista, Boyer-Chammard, and Vivier received funding from innate pharma. Drs. Demaria, Karakunnel, Fares, Batista, Rotolo, Viotti, Boyer-Chammard, and Vivier disclosed that they are employees of Innate Pharma. Dr. Karakunnel received funding from Primevax Precision Biologics. Dr. Rotolo received funding from Sanofi. Dr. Viotti disclosed work for hire. Dr. Lacombe received funding from MSD, Gilead, Janssen, and ViiV Healthcare. Dr. Vivier disclosed that he is a cofounder, shareholder, and employee of Innate Pharma and that his spouse is a shareholder of Innate Pharma. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Members of the FORCE Study Group are listed in the Supplementary Appendix (http://links.lww.com/CCM/H213).
Contributor Information
Collaborators: Nicolas Schleinitz, Julien Carvelli, Marc Gainnier, Jérémy Bourenne, Amandine Bichon, Audrey Le Saux, Fouad Bouzana, Antoine Tilmont, Emi Cauchois, Charlotte Coularet, Nicolas Bruder, Lionel Velly, Mikael Ebbo, Véronique Veit, Estelle Jean, Pierre Simeone, Valéry Blasco, Frédéric Vely, Christelle Piperoglou, Bruno Coutard, Boris Pastorino, Maria Saba Villaroel, Emilie Garrido-Pradalie, Kahéna Amichi, Aurélie Larosa, Aurélie Blondelon, Imane Inal, Kahéna Amichi, Jean Dhorne, Frédérique Durieux, Julie Brunet, Anita Cohen, Bénédicte Deluca, Richard Malkoun, Jean Dellamonica, Matthieu Buscot, Clément Saccheri, Michel Carles, Elisa Demonchy, Eric Cua, David Chirio, Johan Courjon, Karine Risso, Marie-Christine Rigault, Loïc Gazoppi, Virginie Salas, Nadège Bouskila, Irit Touitou, Sophie Breaud, Nihed Boughdiri, Guillaume Marrane, Ferhat Meziani, Hamid Merdji, Julie Helms, Alexandra Monier, Julien Demiselle, Louise-Marie Jandeaux, Antoine Studer, Hayat Allam, Léonie Thiebaut, Anne Hutt-Clauss, Erwan Le Dault, Pierre-Yves Cordier, Hélène Savini, Axelle Clerc, Sophie Spadoni, Emilie Javelle, Axelle Clerc, Nassima Chouaki-Benmansour, Patrick Le Garlantezec, Sarah Le Tohic, Jérôme Allardet-Servent, Lucas Benarous, Corinne Madjarian, Assia Aouadenne-Mesbah, Amélie Rognon, Megan Fraisse, Gaétan Plantefeve, Nasro Benrezzak, Emeline Dubief, Olivia Chauvel, Charlotte Jamet, Saber Davide Barbar, Audrey Ambert, Sophie Lloret, Loubna Elotmani, Grégory Dubois, Séverine Meyrieux, Laurie Barthelemi, Samuel Lehingue, Antoine Poulet, Kristina Bezirganyan, Belkacem Asselate, Vincent Provitolo, Karine Lacombe, Diane Bollens, Cyrielle Letaillandier, Christian Tran, Manuela Sebire, Julie Lamarque, Anne Deguenel-Nguyen, Maxime Desgrouas, Sophie Jacquier, Grégoire Muller, Anne Bretagnol, Armelle Mathonnet, Dalila Benzekri, François Barbier, Nay Mai-Anh, Isabelle Runge, Toufik Kamel, Lucie Muller, Sophie Tellec, Christophe Guervilly, Laurent Papazian, Jean-Marie Forel, Céline Sanz, Camille Pinglis, Sabine Valera, Nathalie Colombini, Fabrice Camou, Gaelle Mourissoux, Olivier Guisset, Nahéma Issa, Delphine Pedenon-Peyrichout, Jean Delaune, Claire Langlade, Stéphane Pedeboscq, Xavier Lescure, Valentina Isernia, Antoine Bachelard, Odile Fleurot, Sylvie Le Gac, Olivia Da Conceicao, Zelie Julia, Lynda Chalal, Lynda Oualit, Laura Kramer, Jennifer Le Grand, Julien Poissy, Saadalla Nseir, Laure Mariller, Claire Delcoutre, Sylvie Brice, Jean-Daniel Lelievre, Sébastien Gallien, Christine Cotellon, William Vindrios, Giovanna Melica, Pierre-André Natella, Marion Bourhis, Alaki Thiemele, Delphine Lefebvre De Nailly, Denis Malvy, Arnaud Desclaux, Mailys Ducours, Pauline Perreau, Alexandre Boyer, Benjamin Clouzeau, Hoang-Nam Bui, Nicolas Bourgoin, Anna Kabala, Bellabes Ghezzoul, Vincent Servant, and Sarah Djabarouti
REFERENCES
- 1.World Health Organization: WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int. Accessed September 28, 2022
- 2.Piroth L, Cottenet J, Mariet AS, et al. : Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021; 9:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez MA, Vuagnat A, Grimaud O, et al. : Impact of ICU transfers on the mortality rate of patients with COVID-19: Insights from comprehensive national database in France. Ann Intensive Care. 2021; 11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RA, Morales-Nebreda L, Markov NS, et al. ; NU SCRIPT Study Investigators: Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021; 590:635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delorey TM, Ziegler CGK, Heimberg G, et al. : COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021; 595:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rendeiro AF, Ravichandran H, Bram Y, et al. : The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021; 593:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan H, Peto R, Henao-Restrepo AM, et al. : Repurposed antiviral drugs for covid-19 — interim WHO solidarity trial results. N Engl J Med. 2021; 384:497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021; 397:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators: Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriazopoulou E, Poulakou G, Milionis H, et al. : Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat Med. 2021; 27:1752–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marconi VC, Ramanan AV, de Bono S, et al. ; COV-BARRIER Study Group: Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021; 9:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guimarães PO, Quirk D, Furtado RH, et al. : Tofacitinib in patients hospitalized with Covid-19 pneumonia N Engl J Med. 2021; 385:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbonell R, Urgelés S, Rodríguez A, et al. ; COVID-19 SEMICYUC Working Group: Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg Health Eur. 2021; 11:100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan B, Freiwald T, Chauss D, et al. : SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 2021; 6:eabg0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen B, Yi X, Sun Y, et al. : Proteomic and metabolomic characterization of covid-19 patient sera. Cell. 2020; 182:59–72.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramlall V, Thangaraj PM, Meydan C, et al. : Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat Med. 2020; 26:1609–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosmann M, Ward PA: Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012; 946:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russkamp NF, Ruemmler R, Roewe J, et al. : Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): A role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J. 2015; 29:3762–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvelli J, Demaria O, Vély F, et al. ; Explore COVID-19 IPH group; Explore COVID-19 Marseille Immunopole group: Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020; 588:146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlaar APJ, de Bruin S, Busch M, et al. : Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): An exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020; 2:e764–e773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annane D, Heming N, Grimaldi-Bensouda L, et al. ; Garches COVID 19 Collaborative Group: Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClinicalMedicine. 2020; 28:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yehya N, Harhay MO, Curley MAQ, et al. : Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019; 200:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ristl R, Frommlet F, Koch A, et al. : Fallback tests for co-primary endpoints. Stat Med. 2016; 35:2669–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali YM, Ferrari M, Lynch NJ, et al. : Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front Immunol. 2021; 12:714511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Yuan X, Chen H, et al. : Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020; 136:2080–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021; 47:60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della-Torre E, Criscuolo E, Lanzillotta M, et al. ; COVID-BioB study group: IL-1 and IL-6 inhibition affects the neutralising activity of anti-SARS-CoV-2 antibodies in patients with COVID-19. Lancet Rheumatol. 2021; 3:e829–e831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gangneux J-P, Dannaoui E, Fekkar A, et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir Med. 2021; S2213-2600:00442–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.